Alan Rapraeger is now retired.
I was a professor in the Department of Human Oncology and the executive director of the UW–Madison Office of Postdoctoral Studies. Members of my laboratory seek to discover novel signaling mechanisms through which cell adhesion regulates the activation and signaling of receptor tyrosine kinases in cancer and to develop new therapeutics that target these mechanisms. Our focus is on the syndecan (Sdc) family of cell-matrix receptors. The syndecans act as organizers of receptor complexes containing integrins and receptor tyrosine kinases (EGFR, IGF1R, VEGFR2 and others) that drive tumorigenesis and tumor angiogenesis. We are developing synstatins (SSTNs), therapeutic peptides that are highly effective inhibitors of these processes in vitro and in vivo and block tumor growth, survival and invasion and the angiogenesis upon which tumors depend.
Education
Postdoctoral Scholar, Stanford University, Developmental Biology (1985)
PhD, University of California–Berkeley, Zoology/Developmental Biology (1978)
BS, University of Oregon, Biology (1972)
Academic Appointments
Professor, Human Oncology (2010)
Professor, Pathology and Laboratory Medicine (1996)
Associate Professor, Pathology and Laboratory Medicine (1991)
Assistant Professor, Pathology and Laboratory Medicine (1985)
Boards, Advisory Committees and Professional Organizations
Executive Director, UW-Madison Office of Postdoctoral Studies (2012–present)
Co-Organizer, International syndecan meeting: “Syndecans in Cell Regulation and Disease," Leuven, Belgium
Vice Chair (2004) and Chair (2006) Proteoglycan Gordon Conference
Steering committee, American Society for Matrix Biology (2005–2008)
Program Leader, Cell Signaling and Growth Control, Carbone Comprehensive Cancer (1997–2001)
Research Focus
Breast Cancer, Multiple Myeloma, Head and Neck Cancer, Immunotherapy
Dr. Alan Rapraeger is a cancer biologist who seeks to discover novel signaling mechanisms through which cell adhesion regulates the activation and signaling of receptor tyrosine kinases in cancer and to develop new therapeutics that target these mechanisms.
The Rapraeger Lab seeks to develop new therapeutics that target receptor tyrosine kinases linked to cell adhesion signaling mechanisms in cancer.
Our focus is on the syndecans (Sdc) family of cell-matrix receptors that act as organizers of receptor complexes containing integrins and receptor tyrosine kinases (EGFR, IGF1R, VEGFR2 and others) that drive tumorigenesis and angiogenesis. We are developing synstatins (SSTNs)—therapeutic peptides that disrupt these signaling mechanisms and block tumor growth, survival and invasion, as well as tumor-induced angiogenesis.
The Rapraeger Lab was the first to demonstrate that syndecans contain “cell binding” motifs in their extracellular domains. The importance of this finding soon became clear when it was realized that such sites are critical for the signaling by growth factor receptor tyrosine kinases (RTKs) in tumor cells. RTKs and integrins have well-established roles in tumor cell proliferation, invasion and survival, often functioning in a coordinated fashion at sites of cell-matrix adhesion. Work from the Rapraeger Lab has shown that syndecans, a four-member class of heparan-sulfate-decorated matrix receptors (Sdc1, Sdc2, Sdc3 and Sdc4), utilize docking motifs in their extracellular domains to organize RTKs and integrins into functional units. This work, combined with prior work from the laboratory that demonstrated as essential role for heparan sulfate (HS) glycosaminoglycans in the activity of growth factors, paints the picture of syndecans as “organizers” of RTK signaling at the cell surface. At present, the laboratory has identified docking motifs that organize signaling apparatus containing EGFR, IGF-1R, VEGFR2 or HER2 and the avb3, avb5, a4b1, a3b1, or a6b4 integrins and the signaling mechanisms activated by these receptor complexes. Peptide mimetics of the docking motifs in the syndecans, called “synstatins” (SSTNIGF1R, SSTNVEGFR2, SSTNVLA-4, SSTNEGFR and SSTNHER2) prevent assembly of the receptor apparatus, block their signaling activities and are highly effective against tumor cell proliferation, survival and invasion. They are also effective against angiogenesis upon which tumors depend. Patents for these peptides have been granted or are pending and work is proceeding to advance them for FDA approval and clinical trials.
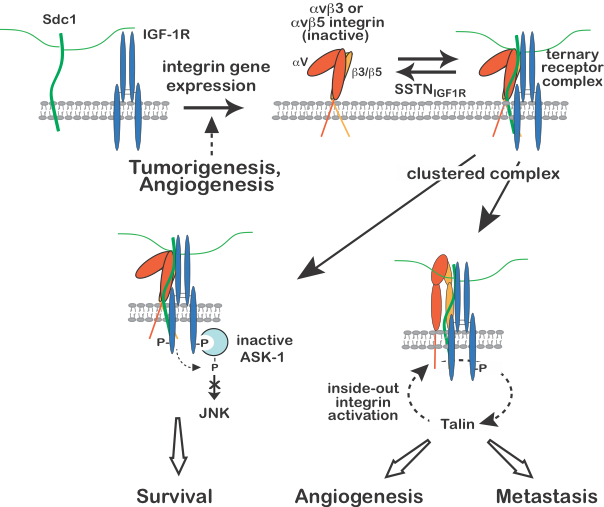
Regulation of IGF-1R signaling by Sdc1.
Expression of the avb3 or avb5 integrin, usually in response to malignant transformation of epithelial cells or activation of endothelial cells during angiogenesis, results in integrin docking to the extracellular domain of human Sdc1 (amino acids 89-120), which is followed by capture of the type 1 insulin-like growth factor receptor (IGF-1R) at the same docking site. Once formed, constitutive or matrix-induced clustering of the ternary receptor complex activates IGF-1R by autophosphorylation independent of IGF-1 growth factor ligand. Activated endothelial cells or tumor cells bearing the ternary receptor complex rely on the syndecan-activated IGF-1R to phosphorylate and suppress the activity of apoptosis signal-regulating kinase-1 (ASK-1) engaged with its cytoplasmic domain, preventing ASK-1-mediated activation of Jun-N-terminal kinase (JNK) and blocking entry into apoptosis, thus sustaining tumor cell survival. In a second activity, downstream signaling from IGF-1R activates the avb3 or avb5 integrin via an inside-out signaling pathway that targets the integrin-activating protein talin, resulting in endothelial or tumor cell motility during the onset of angiogenesis or tumor cell invasion. SSTNIGF1R, a peptide mimetic of the docking site in Sdc1, competitively disrupts the ternary receptor complex on tumor cells and activated endothelial cells, prevents integrin activation and removes the block to ASK-1 activation, inducing tumor cell death and blocking tumor-induced angiogenesis. Neither activity can be rescued by IGF-1 when the receptor complex is disrupted by SSTNIGF1R, emphasizing the singular role played by the syndecan in this IGF-1R signaling mechanism. Work is currently underway examining the integrin activation mechanism, and SSTNIGF1R-mediated inhibition of tumor growth in mouse models of breast and head & neck cancer, and against human patient-derived head & neck cancer xenografts.
Relevant publications
- Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor.
- Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation.
- Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis.
- Synstatin: a selective inhibitor of the syndecan-1-coupled IGF1R-αvβ3 integrin complex in tumorigenesis and angiogenesis.
- Syndecan-1 (CD138) suppresses apoptosis in multiple myeloma by activating IGF1 Receptor: prevention by synstatinIGF1R inhibits tumor growth.
Funding
- NIH R01-CA212413 Syndecan-1 (CD138) and its synstatins: targeting invasion, survival and angiogenesis in myeloma
- NIH 1 P50-DE026787 Project 3: Targeting Head and Neck Cancer with Synstatin Therapeutics
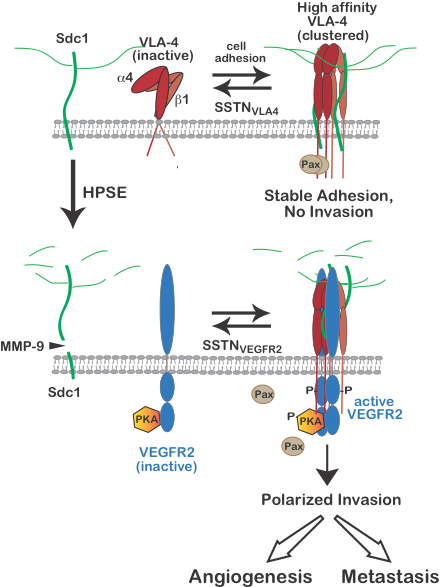
Regulation of VLA-4 activation and VEGFR2-mediated polarized cell invasion of myeloma, melanoma, T-lymphoma and vascular endothelial cells.
A juxtamembrane site in the extracellular domain of human Sdc1 (amino acids 210-236) is responsible for engaging very late antigen-4 (VLA-4, also known as the a4b1 integrin) and vascular endothelial growth factor receptor-2 (VEGFR2), respectively. Synstatin peptide mimetics of the VLA-4 or VEGFR2 binding motifs (SSTNVLA-4 or SSTNVEGFR2), prevent VLA-4 or VEGFR2 capture by the syndecan and disrupt invasion signaling that relies on the co-capture of both receptors. VLA-4 undergoes rapid activation when engaged to ligand, involving a conformation change and clustering (avidity modulation) to increase binding affinity. On cells expressing Sdc1 and VLA-4 (e.g., myeloma cells, vascular endothelial cells, melanoma, Jurkat-T cells and others), this activation is blocked by preventing VLA-4 docking with Sdc1 using SSTNVLA-4 or mutating the VLA-4 binding motif in the syndecan. The mechanism underlying the syndecan-dependent integrin activation mechanism is currently under investigation. In addition to carrying out cell-matrix adhesion, VLA-4 activated by this mechanism becomes localized to the leading edge of the cells where it is highly involved in cell invasion when the cells express the heparan-sulfate-degrading enzyme heparanase. Heparanase is a known tumor promoter and enhancer of leukocyte recruitment during inflammation. Trimming of the HS chains on Sdc1 exposes its core protein to cleavage by matrix-metalloproteinase-9 (MMP-9), releasing the syndecan ectodomain. The VLA-4 and VEGFR2 docking motif in the shed syndecan ectodomain couples VEGFR2 to the clustered integrin, causing VEGF-independent activation of VEGFR2. VEGFR2 signaling leads to activation of protein kinase-A (PKA) engaged with the VEGFR2 cytoplasmic domain and phosphorylation of the a4-integrin cytoplasmic domain on serine 988. This leads to Rac1 activation, causing lamellipodium formation, establishment of a leading edge, and polarized invasion of tumor cells and vascular endothelial cells necessary for tumor cell metastasis and angiogenesis. Either prevention of integrin activation by SSTNVLA-4 or VEGFR2-coupling to Sdc4 by SSTNVEGFR2 serves to block these processes. Work is currently under way examining these processes and the efficacy of the SSTNs in mouse models of multiple myeloma and immune suppression.
Relevant publications
- Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins.
- Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy.
Funding
- NIH R01-CA212413 Syndecan-1 (CD138) and its synstatins: targeting invasion, survival and angiogenesis in myeloma
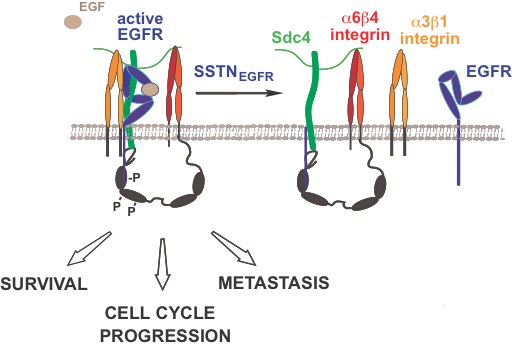
Coupling of EGFR to integrins by Sdc4 regulates tumor cell proliferation, survival and invasion.
A juxtamembrane site in human Sdc4 (amino acids 87-131) captures the epidermal growth factor receptor (EGFR) and the a3b1 integrin. In addition, the C-terminus of the syndecan engages the cytoplasmic C-terminus of the b4 integrin, which comprises the a6b4 integrin. Several tyrosines in the b4 cytoplasmic “signaling domain” become phosphorylated, and the receptor complex as a whole supports the proliferation, survival and invasion of tumor cells (breast carcinoma, head & neck squamous cell carcinoma) and endothelial cells engaged in pathological angiogenesis. Ongoing work suggests that the receptor complex acts to suppress stress signaling and to promote cell cycle progression in the tumor cells, evidenced by the findings that an inhibitory synstatin mimetic of the docking site in Sdc4 (SSTNEGFR) causes rapid activation of stress MAPKs (p38MAPK and JNK), and rapid G1 and S-phase cell cycle arrest specifically in tumor cells. Ongoing work is examining the mechanism of cell cycle arrest in breast and head & neck cancer, and the mechanism of EGFR activation and signaling when coupled to the syndecan.
Relevant publications
- Interaction of syndecan and alpha6beta4 integrin cytoplasmic domains: regulation of ErbB2-mediated integrin activation.
- Cytoplasmic domain interactions of syndecan-1 and syndecan-4 with α6β4 integrin mediate human epidermal growth factor receptor (HER1 and HER2)-dependent motility and survival.
- Syndecan-1 and syndecan-4 capture EGFR family members and the α3β1 integrin via binding sites in their ectodomains: novel synstatins prevent kinase capture and inhibit α6β4-integrin-dependent epithelial cell motility.
Funding
- NIH R01-CA163662 Role of Syndecans in HER2 and TN breast cancer
- NIH 1 P50-DE026787 Project 3: Targeting Head and Neck Cancer with Synstatin Therapeutics
Alan Rapraeger is retiring on March 25.
He and his lab will be missed.
-
Inhibiting IGF1R-mediated Survival Signaling in Head and Neck Cancer with the Peptidomimetic SSTN<sub>IGF1R</sub> Cancer research communications
Stueven NA, Beauvais DM, Hu R, Kimple RJ, Rapraeger AC
2023 Jan 19;3(1):97-108. doi: 10.1158/2767-9764.CRC-22-0274. eCollection 2023 Jan.
-
More
Previous studies have shown that the type I IGFR (IGF1R) suppresses apoptosis when it is autoactivated by coupling its extracellular domain to a matrix adhesion receptor complex consisting of syndecan-1 (Sdc1) and αvβ3 or αvβ5 integrin. We now report that head and neck squamous cell carcinoma (HNSCC) relies on this receptor complex. Disruption of the complex in HNSCC cells in vitro with a peptide mimetic of the organizer site in Sdc1 (called SSTNIGF1R) inactivates IGF1R, even in the presence of IGF1, and relieves the suppression of apoptosis signal-regulating kinase-1 (ASK1), dramatically reducing tumor cell survival. Normal epithelial cells do not assemble this receptor complex, require IGF1 to activate the IGF1R, and are refractory to SSTNIGF1R. In vivo, SSTNIGF1R reduced the growth of patient-derived HNSCC tumors in immunodeficient mice by 85%-95%. IGF1R's assimilation into the matrix receptor complex, which is detected in these tumors using the proximity ligation assay (PLA), is quantitatively disrupted by SSTNIGF1R, coinciding with ASK1 activation. PLA also detects the IGF1R-containing receptor complex in the archival sections of tonsil carcinomas, whereas the adjacent benign epithelium is negative. Likewise, PLA screening of oropharyngeal and adenoid cystic tumor microarrays demonstrated that over 95% of the tumors contained this unique receptor complex with no detectable expression in benign tissue. These findings suggest that HNSCC upregulates and is highly dependent on IGF1R signaling via this adhesion receptor complex. Targeting this mechanism with novel therapeutics, including highly specific SSTNIGF1R, is likely to offer promising outcomes for patients with carcinoma.
SIGNIFICANCE: A newly developed biomarker reveals upregulation of an antiapoptotic IGF1R-integrin-syndecan receptor complex in head and neck cancer and documents disruption of the complex in patient-derived tumor xenografts (PDX) treated with the inhibitor SSTNIGF1R. A corresponding blockade in PDX growth in the presence of this inhibitor demonstrates that therapies designed to target this mechanism will likely offer promising outcomes for patients with head and neck cancer.
PMID:36968227 | PMC:PMC10035507 | DOI:10.1158/2767-9764.CRC-22-0274
View details for PubMedID 36968227
-
More
-
Ponatinib sensitizes myeloma cells to MEK inhibition in the high-risk VQ model Scientific reports
Flietner E, Wen Z, Rajagopalan A, Jung O, Watkins L, Wiesner J, You X, Zhou Y, Sun Y, Kingstad-Bakke B, Callander NS, Rapraeger A, Suresh M, Asimakopoulos F, Zhang J
2022 Jun 23;12(1):10616. doi: 10.1038/s41598-022-14114-z.
-
More
Multiple myeloma (MM) is a malignant plasma cell cancer. Mutations in RAS pathway genes are prevalent in advanced and proteasome inhibitor (PI) refractory MM. As such, we recently developed a VQ MM mouse model recapitulating human advanced/high-risk MM. Using VQ MM cell lines we conducted a repurposing screen of 147 FDA-approved anti-cancer drugs with or without trametinib (Tra), a MEK inhibitor. Consistent with its high-risk molecular feature, VQ MM displayed reduced responses to PIs and de novo resistance to the BCL2 inhibitor, venetoclax. Ponatinib (Pon) is the only tyrosine kinase inhibitor that showed moderate MM killing activity as a single agent and strong synergism with Tra in vitro. Combined Tra and Pon treatment significantly prolonged the survival of VQ MM mice regardless of treatment schemes. However, this survival benefit was moderate compared to that of Tra alone. Further testing of Tra and Pon on cytotoxic CD8+ T cells showed that Pon, but not Tra, blocked T cell function in vitro, suggesting that the negative impact of Pon on T cells may partially counteract its MM-killing synergism with Tra in vivo. Our study provides strong rational to comprehensively evaluate agents on both MM cells and anti-MM immune cells during therapy development.
PMID:35739276 | PMC:PMC9226136 | DOI:10.1038/s41598-022-14114-z
View details for PubMedID 35739276
-
More
-
Plasma membrane proteoglycans syndecan-2 and syndecan-4 engage with EGFR and RON kinase to sustain carcinoma cell cycle progression The Journal of biological chemistry
Beauvais DM, Nelson SE, Adams KM, Stueven NA, Jung O, Rapraeger AC
2022 Jun;298(6):102029. doi: 10.1016/j.jbc.2022.102029. Epub 2022 May 13.
-
More
Epidermal growth factor receptor (EGFR) is a causal factor in carcinoma, yet many carcinoma patients are resistant to EGFR inhibitors. Potential insight into this resistance stems from prior work that showed EGFR in normal epithelial cells docks to the extracellular domain of the plasma membrane proteoglycan syndecan-4 (Sdc4) engaged with α3β1 and α6β4 integrins. We now report that this receptor complex is modified by the recruitment of syndecan-2 (Sdc2), the Recepteur d'Origine Nantais (RON) tyrosine kinase, and the cellular signaling mediator Abelson murine leukemia viral oncogene homolog 1 (ABL1) in triple-negative breast carcinoma and head and neck squamous cell carcinoma, where it contributes to EGFR kinase-independent proliferation. Treatment with a peptide mimetic of the EGFR docking site in the extracellular domain of Sdc4 (called SSTNEGFR) disrupts the entire complex and causes a rapid, global arrest of the cell cycle. Normal epithelial cells do not recruit these additional receptors to the adhesion mechanism and are not arrested by SSTNEGFR. Although EGFR docking with Sdc4 in the tumor cells is required, cell cycle progression does not depend on EGFR kinase. Instead, progression depends on RON kinase, activated by its incorporation into the complex. RON activates ABL1, which suppresses p38 mitogen-activated protein kinase and prevents a p38-mediated signal that would otherwise arrest the cell cycle. These findings add to the growing list of receptor tyrosine kinases that support tumorigenesis when activated by their association with syndecans at sites of matrix adhesion and identify new potential targets for cancer therapy.
PMID:35569509 | PMC:PMC9190016 | DOI:10.1016/j.jbc.2022.102029
View details for PubMedID 35569509
-
More
-
Syndecans and Their Synstatins: Targeting an Organizer of Receptor Tyrosine Kinase Signaling at the Cell-Matrix Interface Frontiers in oncology
Rapraeger AC
2021 Oct 27;11:775349. doi: 10.3389/fonc.2021.775349. eCollection 2021.
-
More
Receptor tyrosine kinases (RTKs) and integrin matrix receptors have well-established roles in tumor cell proliferation, invasion and survival, often functioning in a coordinated fashion at sites of cell-matrix adhesion. Central to this coordination are syndecans, another class of matrix receptor, that organize RTKs and integrins into functional units, relying on docking motifs in the syndecan extracellular domains to capture and localize RTKs (e.g., EGFR, IGF-1R, VEGFR2, HER2) and integrins (e.g., αvβ3, αvβ5, α4β1, α3β1, α6β4) to sites of adhesion. Peptide mimetics of the docking motifs in the syndecans, called "synstatins", prevent assembly of these receptor complexes, block their signaling activities and are highly effective against tumor cell invasion and survival and angiogenesis. This review describes our current understanding of these four syndecan-coupled mechanisms and their inhibitory synstatins (SSTNIGF1R, SSTNVEGFR2, SSTNVLA-4, SSTNEGFR and SSTNHER2).
PMID:34778093 | PMC:PMC8578902 | DOI:10.3389/fonc.2021.775349
View details for PubMedID 34778093
-
More
-
VLA-4 phosphorylation during tumor and immune cell migration relies on its coupling to VEGFR2 and CXCR4 by syndecan-1 Journal of cell science
Jung O, Beauvais DM, Adams KM, Rapraeger AC
2019 Oct 28;132(20):jcs232645. doi: 10.1242/jcs.232645.
-
More
When targeted by the tumor-promoting enzyme heparanase, cleaved and shed syndecan-1 (Sdc1) then couples VEGFR2 (also known as KDR) to VLA-4, activating VEGFR2 and the directed migration of myeloma cells. But how VEGFR2 activates VLA-4-mediated motility has remained unknown. We now report that VEGFR2 causes PKA-mediated phosphorylation of VLA-4 on S988, an event known to stimulate tumor metastasis while suppressing cytotoxic immune cells. A key partner in this mechanism is the chemokine receptor CXCR4, a well-known mediator of cell motility in response to gradients of the chemokine SDF-1 (also known as CXCL12). The entire machinery necessary to phosphorylate VLA-4, consisting of CXCR4, AC7 (also known as ADCY7) and PKA, is constitutively associated with VEGFR2 and is localized to the integrin by Sdc1. VEGFR2 carries out the novel phosphorylation of Y135 within the DRY microswitch of CXCR4, sequentially activating Gαiβγ, AC7 and PKA, which phosphorylates S988 on the integrin. This mechanism is blocked by a syndecan-mimetic peptide (SSTNVEGFR2), which, by preventing VEGFR2 linkage to VLA-4, arrests tumor cell migration that depends on VLA-4 phosphorylation and stimulates the LFA-1-mediated migration of cytotoxic leukocytes.
PMID:31562188 | PMC:PMC6826009 | DOI:10.1242/jcs.232645
View details for PubMedID 31562188
-
More
-
The Specificity of EGF-Stimulated IQGAP1 Scaffold Towards the PI3K-Akt Pathway is Defined by the IQ3 motif Scientific reports
Chen M, Choi S, Jung O, Wen T, Baum C, Thapa N, Lambert PF, Rapraeger AC, Anderson RA
2019 Jun 24;9(1):9126. doi: 10.1038/s41598-019-45671-5.
-
More
Epidermal growth factor receptor (EGFR) and its downstream phosphoinositide 3-kinase (PI3K) pathway are commonly deregulated in cancer. Recently, we have shown that the IQ motif-containing GTPase-activating protein 1 (IQGAP1) provides a molecular platform to scaffold all the components of the PI3K-Akt pathway and results in the sequential generation of phosphatidylinositol-3,4,5-trisphosphate (PI3,4,5P3). In addition to the PI3K-Akt pathway, IQGAP1 also scaffolds the Ras-ERK pathway. To define the specificity of IQGAP1 for the control of PI3K signaling, we have focused on the IQ3 motif in IQGAP1 as PIPKIα and PI3K enzymes bind this region. An IQ3 deletion mutant loses interactions with the PI3K-Akt components but retains binding to ERK and EGFR. Consistently, blocking the IQ3 motif of IQGAP1 using an IQ3 motif-derived peptide mirrors the effect of IQ3 deletion mutant by reducing Akt activation but has no impact on ERK activation. Also, the peptide disrupts the binding of IQGAP1 with PI3K-Akt pathway components, while IQGAP1 interactions with ERK and EGFR are not affected. Functionally, deleting or blocking the IQ3 motif inhibits cell proliferation, invasion, and migration in a non-additive manner to a PIPKIα inhibitor, establishing the functional specificity of IQ3 motif towards the PI3K-Akt pathway. Taken together, the IQ3 motif is a specific target for suppressing activation of the PI3K-Akt but not the Ras-ERK pathway. Although EGFR stimulates the IQGAP1-PI3K and -ERK pathways, here we show that IQGAP1-PI3K controls migration, invasion, and proliferation independent of ERK. These data illustrate that the IQ3 region of IQGAP1 is a promising therapeutic target for PI3K-driven cancer.
PMID:31235839 | PMC:PMC6591252 | DOI:10.1038/s41598-019-45671-5
View details for PubMedID 31235839
-
More
-
The Hidden Conundrum of Phosphoinositide Signaling in Cancer Trends in cancer
Thapa N, Tan X, Choi S, Lambert PF, Rapraeger AC, Anderson RA
2016 Jul;2(7):378-390. doi: 10.1016/j.trecan.2016.05.009. Epub 2016 Jun 20.
-
More
Phosphoinositide 3-kinase (PI3K) generation of PI(3,4,5)P3 from PI(4,5)P2 and the subsequent activation of Akt and its downstream signaling cascades (e.g. mTORC1) dominates the landscape of phosphoinositide signaling axis in cancer research. However, PI(4,5)P2 is breaking its boundary as merely a substrate for PI3K and phospholipase C (PLC), and is now an established lipid messenger pivotal for different cellular events in cancer. Here, we review the phosphoinositide signaling axis in cancer, giving due weight to PI(4,5)P2 and its generating enzymes, the phosphatidylinositol phosphate (PIP) kinases (PIPKs). We highlighted how PI(4,5)P2 and PIP kinases serve as a proximal node in phosphoinositide signaling axis and how its interaction with cytoskeletal proteins regulates migratory and invasive nexus of metastasizing tumor cells.
PMID:27819060 | PMC:PMC5094282 | DOI:10.1016/j.trecan.2016.05.009
View details for PubMedID 27819060
-
More
-
Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy The FEBS journal
Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I
2017 Jan;284(1):42-55. doi: 10.1111/febs.13932. Epub 2016 Nov 16.
-
More
Because of its impact on multiple biological pathways, heparanase has emerged as a major regulator of cancer, inflammation and other disease processes. Heparanase accomplishes this by degrading heparan sulfate which regulates the abundance and location of heparin-binding growth factors thereby influencing multiple signaling pathways that control gene expression, syndecan shedding and cell behavior. In addition, heparanase can act via nonenzymatic mechanisms that directly activate signaling at the cell surface. Clinical trials testing heparanase inhibitors as anticancer therapeutics are showing early signs of efficacy in patients further emphasizing the biological importance of this enzyme. This review focuses on recent developments in the field of heparanase regulation of cancer and inflammation, including the impact of heparanase on exosomes and autophagy, and novel mechanisms whereby heparanase regulates tumor metastasis, angiogenesis and chemoresistance. In addition, the ongoing development of heparanase inhibitors and their potential for treating cancer and inflammation are discussed.
PMID:27758044 | PMC:PMC5226874 | DOI:10.1111/febs.13932
View details for PubMedID 27758044
-
More
-
Syndecan-1 (CD138) Suppresses Apoptosis in Multiple Myeloma by Activating IGF1 Receptor: Prevention by SynstatinIGF1R Inhibits Tumor Growth Cancer research
Beauvais DM, Jung O, Yang Y, Sanderson RD, Rapraeger AC
2016 Sep 1;76(17):4981-93. doi: 10.1158/0008-5472.CAN-16-0232. Epub 2016 Jun 30.
-
More
Syndecan-1 (Sdc1/CD138) expression is linked to disease severity in multiple myeloma, although the causal basis for this link remains unclear. Here we report that capture of the IGF1 receptor (IGF1R) by Sdc1 suppresses ASK1-dependent apoptosis in multiple myeloma cells. Sdc1 binds two different fractions of IGF1R, one that is constitutively active and a second that is activated by IGF1 ligand. Notably, IGF1R kinase activity in both fractions is blocked by synstatinIGF1R (SSTNIGF1R), a peptide that inhibits IGF1R capture by Sdc1, as well as by a truncated peptide (SSTNIGF1R-T) that appears to be specific for multiple myeloma cells. Mechanistically, we show that ASK1 is bound to active IGF1R and inhibited by Tyr and Ser83/Ser966 phosphorylation. When IGF1R engagement with Sdc1 is blocked by SSTNIGF1R, ASK1 becomes activated, and initiates JNK- and caspase-3-mediated apoptosis. In pharmacologic tests, we find SSTNIGF1R is highly stable in human plasma and displays a half-life of 27 hours in mice, wherein it significantly reduces both the size and neovascularization of CAG myeloma tumor xenografts. Taken together, our results offer a preclinical proof of concept and mechanistic rationale for the exploration of SSTNIGF1R as an experimental therapeutic to dually attack multiple myeloma tumor cell survival and tumor angiogenesis. Cancer Res; 76(17); 4981-93. ©2016 AACR.
PMID:27364558 | PMC:PMC5010496 | DOI:10.1158/0008-5472.CAN-16-0232
View details for PubMedID 27364558
-
More
-
Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins Oncogenesis
Jung O, Trapp-Stamborski V, Purushothaman A, Jin H, Wang H, Sanderson RD, Rapraeger AC
2016 Feb 29;5(2):e202. doi: 10.1038/oncsis.2016.5.
-
More
Multiple myeloma arises when malignant plasma cells invade and form multiple tumors in the bone marrow. High levels of heparanase (HPSE) correlate with poor prognosis in myeloma patients. A likely target of the enzyme is the heparan sulfate (HS) proteoglycan syndecan-1 (Sdc1, CD138), which is highly expressed on myeloma cells and contributes to poor prognosis in this disease. We find that HPSE promotes an invasive phenotype mediated by the very late antigen-4 (VLA-4, or α4β1 integrin) in myeloma cells plated on either fibronectin (FN) or vascular endothelial cell adhesion molecule-1 (VCAM-1), ligands that are prevalent in the bone marrow. The phenotype depends on vascular endothelial cell growth factor receptor-2 (VEGFR2), which is aberrantly expressed in myeloma, and is characterized by a highly protrusive lamellipodium and cell invasion. HPSE-mediated trimming of the HS on Sdc1 and subsequent matrix metalloproteinase-9-mediated shedding of the syndecan exposes a juxtamembrane site in Sdc1 that binds VEGFR2 and VLA-4, thereby coupling VEGFR2 to the integrin. Shed Sdc1 can be mimicked by recombinant Sdc1 ectodomain or by a peptide based on its binding motif, which causes VLA-4 to re-orient from the lagging edge (uropod) to the leading edge of migrating cells, couple with and activate VEGFR2. Peptides (called 'synstatins') containing only the VLA-4 or VEGFR2 binding sites competitively inhibit invasion, as they block coupling of the receptors. This mechanism is also utilized by vascular endothelial cells, in which it is also activated by HPSE, during endothelial cell tube formation. Collectively, our findings reveal for the first time the mechanism through which HPSE modulates Sdc1 function to promote both tumor cell invasion and angiogenesis, thereby driving multiple myeloma progression. The inhibitory synstatins, or inhibitors of HPSE enzyme activity, are likely to show promise as therapeutics against myeloma extravasation and spread.
PMID:26926788 | PMC:PMC5154350 | DOI:10.1038/oncsis.2016.5
View details for PubMedID 26926788
-
More
-
Stress-Induced EGFR Trafficking: Mechanisms, Functions, and Therapeutic Implications Trends in cell biology
Tan X, Lambert PF, Rapraeger AC, Anderson RA
2016 May;26(5):352-366. doi: 10.1016/j.tcb.2015.12.006. Epub 2016 Jan 27.
-
More
Epidermal growth factor receptor (EGFR) has fundamental roles in normal physiology and cancer, making it a rational target for cancer therapy. Surprisingly, however, inhibitors that target canonical, ligand-stimulated EGFR signaling have proven to be largely ineffective in treating many EGFR-dependent cancers. Recent evidence indicates that both intrinsic and therapy-induced cellular stress triggers robust, noncanonical pathways of ligand-independent EGFR trafficking and signaling, which provides cancer cells with a survival advantage and resistance to therapeutics. Here, we review the mechanistic regulation of noncanonical EGFR trafficking and signaling, and the pathological and therapeutic stresses that activate it. We also discuss the implications of this pathway in clinical treatment of EGFR-overexpressing cancers.
PMID:26827089 | PMC:PMC5120732 | DOI:10.1016/j.tcb.2015.12.006
View details for PubMedID 26827089
-
More
-
Syndecan-1 and Syndecan-4 Capture Epidermal Growth Factor Receptor Family Members and the α3β1 Integrin Via Binding Sites in Their Ectodomains: NOVEL SYNSTATINS PREVENT KINASE CAPTURE AND INHIBIT α6β4-INTEGRIN-DEPENDENT EPITHELIAL CELL MOTILITY The Journal of biological chemistry
Wang H, Jin H, Rapraeger AC
2015 Oct 23;290(43):26103-13. doi: 10.1074/jbc.M115.679084. Epub 2015 Sep 8.
-
More
The α6β4 integrin is known to associate with receptor tyrosine kinases when engaged in epithelial wound healing and in carcinoma invasion and survival. Prior work has shown that HER2 associates with α6β4 integrin and syndecan-1 (Sdc1), in which Sdc1 engages the cytoplasmic domain of the β4 integrin subunit allowing HER2-dependent motility and carcinoma cell survival. In contrast, EGFR associates with Sdc4 and the α6β4 integrin, and EGFR-dependent motility depends on cytoplasmic engagement of β4 integrin with Sdc4. However, how HER2 and EGFR assimilate into a complex with the syndecans and integrin, and why kinase capture is syndecan-specific has remained unknown. In the present study, we demonstrate that HER2 is captured via a site, comprised of amino acids 210-240, in the extracellular domain of human Sdc1, and EGFR is captured via an extracellular site comprised of amino acids 87-131 in human Sdc4. Binding assays using purified recombinant proteins demonstrate that the interaction between the EGFR family members and the syndecans is direct. The α3β1 integrin, which is responsible for the motility of the cells, is captured at these sites as well. Peptides based on the interaction motifs in Sdc1 and Sdc4, called synstatins (SSTN210-240 and SSTN87-131) competitively displace the receptor tyrosine kinase and α3β1 integrin from the syndecan with an IC50 of 100-300 nm. The syndecans remain anchored to the α6β4 integrin via its cytoplasmic domain, but the activation of cell motility is disrupted. These novel SSTN peptides are potential therapeutics for carcinomas that depend on these HER2- and EGFR-coupled mechanisms for their invasion and survival.
PMID:26350464 | PMC:PMC4646262 | DOI:10.1074/jbc.M115.679084
View details for PubMedID 26350464
-
More
-
Cytoplasmic domain interactions of syndecan-1 and syndecan-4 with α6β4 integrin mediate human epidermal growth factor receptor (HER1 and HER2)-dependent motility and survival The Journal of biological chemistry
Wang H, Jin H, Beauvais DM, Rapraeger AC
2014 Oct 31;289(44):30318-30332. doi: 10.1074/jbc.M114.586438. Epub 2014 Sep 8.
-
More
Epithelial cells are highly dependent during wound healing and tumorigenesis on the α6β4 integrin and its association with receptor tyrosine kinases. Previous work showed that phosphorylation of the β4 subunit upon matrix engagement depends on the matrix receptor syndecan (Sdc)-1 engaging the cytoplasmic domain of the β4 integrin and coupling of the integrin to human epidermal growth factor receptor-2 (HER2). In this study, HER2-dependent migration activated by matrix engagement is compared with migration stimulated by EGF. We find that whereas HER2-dependent migration depends on Sdc1, EGF-dependent migration depends on a complex consisting of human epidermal growth factor receptor-1 (HER1, commonly known as EGFR), α6β4, and Sdc4. The two syndecans recognize distinct sites at the extreme C terminus of the β4 integrin cytoplasmic domain. The binding motif in Sdc1 is QEEXYX, composed in part by its syndecan-specific variable (V) region and in part by the second conserved (C2) region that it shares with other syndecans. A cell-penetrating peptide containing this sequence competes for HER2-dependent epithelial migration and carcinoma survival, although it is without effect on the EGFR-stimulated mechanism. β4 mutants bearing mutations specific for Sdc1 and Sdc4 recognition act as dominant negative mutants to block cell spreading or cell migration that depends on HER2 or EGFR, respectively. The interaction of the α6β4 integrin with the syndecans appears critical for it to be utilized as a signaling platform; migration depends on α3β1 integrin binding to laminin 332 (LN332; also known as laminin 5), whereas antibodies that block α6β4 binding are without effect. These findings indicate that specific syndecan family members are likely to have key roles in α6β4 integrin activation by receptor tyrosine kinases.
PMID:25202019 | PMC:PMC4215216 | DOI:10.1074/jbc.M114.586438
View details for PubMedID 25202019
-
More
-
Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity The Journal of biological chemistry
Zhang Y, Wang N, Raab RW, McKown RL, Irwin JA, Kwon I, van Kuppevelt H, Laurie GW
2013 Apr 26;288(17):12090-101. doi: 10.1074/jbc.M112.422717. Epub 2013 Mar 15.
-
More
Cell surface heparan sulfate (HS) proteoglycans shape organogenesis and homeostasis by capture and release of morphogens through mechanisms largely thought to exclude the core protein domain. Nevertheless, heparanase deglycanation of the N-terminal HS-rich domain of syndecan-1 (SDC1), but not SDC2 or -4, is a prerequisite for binding of the prosecretory mitogen lacritin (Ma, P., Beck, S. L., Raab, R. W., McKown, R. L., Coffman, G. L., Utani, A., Chirico, W. J., Rapraeger, A. C., and Laurie, G. W. (2006) Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J. Cell Biol. 174, 1097-1106). We now report that the conserved and hydrophobic GAGAL domain in SDC1, adjacent to predicted HS substitution sites, is necessary to ligate and substantially enhance the α-helicity of the amphipathic C terminus of lacritin. Swapping out GAGAL for GADED in SDC2 or for GDLDD in SDC4 (both less hydrophobic) abrogated binding. HS and chondroitin sulfate are also essential. Both are detected in the N terminus, and when incubated with antibodies HS4C3 (anti-HS) or IO3H10 (anti-chondroitin sulfate), binding was absent, as occurred when all three N-terminal glycosaminoglycan substitution sites were mutated to alanine or when cells were treated with 4-methylumbelliferyl-β-d-xylopyranoside or chlorate to suppress glycosaminoglycan substitution or sulfation, respectively. SDC1 interacts with the hydrophobic face of lacritin via Leu-108/Leu-109/Phe-112 as well as with Glu-103/Lys-107 and Lys-111 of the largely cationic face. Carving a hybrid hydrophobic/electrostatic docking site out of SDC1 in a manner dependent on endogenous heparanase is a dynamic process appropriate for subtle or broad epithelial regulation in morphogenesis, health, and disease.
PMID:23504321 | PMC:PMC3636894 | DOI:10.1074/jbc.M112.422717
View details for PubMedID 23504321
-
More
-
Synstatin: a selective inhibitor of the syndecan-1-coupled IGF1R-αvβ3 integrin complex in tumorigenesis and angiogenesis The FEBS journal
Rapraeger AC
2013 May;280(10):2207-15. doi: 10.1111/febs.12160. Epub 2013 Feb 24.
-
More
The syndecans are a family of heparan sulfate-decorated cell-surface proteoglycans: matrix receptors with roles in cell adhesion and growth factor signaling. Their heparan sulfate chains recognize 'heparin-binding' motifs that are ubiquitously present in the extracellular matrix, providing the means for syndecans to constitutively bind and cluster to sites of cell-matrix adhesion. Emerging evidence suggests that specialized docking sites in the syndecan extracellular domains may serve to localize other receptors to these sites as well, including integrins and growth factor receptor tyrosine kinases. A prototype of this mechanism is capture of the αvβ3 integrin and insulin-like growth factor 1 receptor (IGF1R) by syndecan-1 (Sdc1), forming a ternary receptor complex in which signaling downstream of IGF1R activates the integrin. This Sdc1-coupled ternary receptor complex is especially prevalent on tumor cells and activated endothelial cells undergoing angiogenesis, reflecting the up-regulated expression of αvβ3 integrin in such cells. As such, much effort has focused on developing therapeutic agents that target this integrin in various cancers. Along these lines, the site in the Sdc1 ectodomain that is responsible for capture and activation of the αvβ3 or αvβ5 integrins by IGF1R can be mimicked by a short peptide called 'synstatin', which competitively displaces the integrin and IGF1R kinase from the syndecan and inactivates the complex. This review summarizes our current knowledge of the Sdc1-coupled ternary receptor complex and the efficacy of synstatin as an emerging therapeutic agent to target this signaling mechanism.
PMID:23375101 | PMC:PMC3651771 | DOI:10.1111/febs.12160
View details for PubMedID 23375101
-
More
-
Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis The FEBS journal
Rapraeger AC, Ell BJ, Roy M, Li X, Morrison OR, Thomas GM, Beauvais DM
2013 May;280(10):2194-206. doi: 10.1111/febs.12134. Epub 2013 Feb 11.
-
More
Vascular endothelial growth factor (VEGF)-stimulated angiogenesis depends on a cross-talk mechanism involving VEGF receptor 2 (VEGFR2), vascular endothelial (VE)-cadherin and the αVβ3 integrin. Because we have shown that αVβ3 integrin activation is dependent on its incorporation, along with the insulin-like growth factor-1 receptor (IGF1R) kinase, into a ternary receptor complex organized by the matrix receptor syndecan-1 (Sdc1), we questioned the role of this core complex in VEGF-stimulated angiogenesis. We find that the Sdc1-coupled ternary receptor complex is required for VEGF signalling and for stimulation of vascular endothelial cell migration by vascular endothelial cadherin (VE-cadherin) engagement. VE-cadherin binding to Fc/VE-cadherin extracellular domain chimera activates Sdc1-coupled IGF1R and αvβ3 integrin; this depends on VEGFR2 and c-Src activated by the cadherin. Blocking homotypic VE-cadherin engagement disrupts VEGF-stimulated cell migration, which is restored by clustering the cadherin in the absence of cell-cell adhesion. This cadherin-dependent stimulation requires VEGFR2 and IGF1R and is blocked by synstatin (SSTN)(92-119), a peptide that competitively disrupts the Sdc1-coupled ternary complex and prevents the αVβ3 integrin activation required for VEGFR2 activation. VEGFR2-stimulated angiogenesis in the mouse aortic ring explant assay is disrupted by SSTN, although only early in the process, suggesting that IGF1R coupling to Sdc1 and αVβ3 integrin comprises a core activation mechanism activated by VE-cadherin that is necessary for VEGFR2 and integrin activation in the initial stages of endothelial cell dissemination during angiogenesis.
PMID:23331867 | PMC:PMC3640762 | DOI:10.1111/febs.12134
View details for PubMedID 23331867
-
More
-
Transmembrane and extracellular domains of syndecan-1 have distinct functions in regulating lung epithelial migration and adhesion The Journal of biological chemistry
Altemeier WA, Schlesinger SY, Buell CA, Brauer R, Rapraeger AC, Parks WC, Chen P
2012 Oct 12;287(42):34927-34935. doi: 10.1074/jbc.M112.376814. Epub 2012 Aug 30.
-
More
Syndecan-1 is a cell surface proteoglycan that can organize co-receptors into a multimeric complex to transduce intracellular signals. The syndecan-1 core protein has multiple domains that confer distinct cell- and tissue-specific functions. Indeed, the extracellular, transmembrane, and cytoplasmic domains have all been found to regulate specific cellular processes. Our previous work demonstrated that syndecan-1 controls lung epithelial migration and adhesion. Here, we identified the necessary domains of the syndecan-1 core protein that modulate its function in lung epithelial repair. We found that the syndecan-1 transmembrane domain has a regulatory function in controlling focal adhesion disassembly, which in turn controls cell migration speed. In contrast, the extracellular domain facilitates cell adhesion through affinity modulation of α(2)β(1) integrin. These findings highlight the fact that syndecan-1 is a multidimensional cell surface receptor that has several regulatory domains to control various biological processes. In particular, the lung epithelium requires the syndecan-1 transmembrane domain to govern cell migration and is independent from its ability to control cell adhesion via the extracellular domain.
PMID:22936802 | PMC:PMC3471708 | DOI:10.1074/jbc.M112.376814
View details for PubMedID 22936802
-
More
-
Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation Journal of cell science
Beauvais DM, Rapraeger AC
2010 Nov 1;123(Pt 21):3796-807. doi: 10.1242/jcs.067645.
-
More
Syndecan-1 (Sdc1) engages and activates the αvβ3 (and/or αvβ5) integrin when clustered in human carcinoma and endothelial cells. Although the engagement is extracellular, the activation mechanism is cytoplasmic. This talin-dependent, inside-out signaling pathway is activated downstream of the insulin-like growth factor-1 receptor (IGF1R), whose kinase activity is triggered by Sdc1 clustering. In vitro binding assays using purified receptors suggest that association of the Sdc1 ectodomain with the integrin provides a 'docking face' for IGF1R. IGF1R docking and activation of the associated integrin is blocked by synstatin (SSTN(92-119)), a peptide derived from the integrin engagement site in Sdc1. IGF1R colocalizes with αvβ3 integrin and Sdc1 in focal contacts, but fails to associate with or activate the integrin in cells either lacking Sdc1 or expressing Sdc1(Δ67-121), a mutant that is unable to form the Sdc1-integrin-IGF1R ternary complex. Integrin activation is also blocked by IGF1R inhibitors or by silencing IGF1R or talin expression with small-interfering RNAs (siRNAs). In both cases, expression of the constitutively active talin F23 head domain rescues integrin activation. We recently reported that SSTN(92-119) blocks angiogenesis and impairs tumor growth in mice, therefore this Sdc1-mediated integrin regulatory mechanism might be a crucial regulator of disease processes known to rely on these integrins, including tumor cell metastasis and tumor-induced angiogenesis.
PMID:20971705 | PMC:PMC2964108 | DOI:10.1242/jcs.067645
View details for PubMedID 20971705
-
More
-
Focus on molecules: syndecan-1 Experimental eye research
Zhang Y, McKown RL, Raab RW, Rapraeger AC, Laurie GW
2011 Oct;93(4):329-30. doi: 10.1016/j.exer.2010.06.008. Epub 2010 Jun 23.
-
Heparan sulfate domain organization and sulfation modulate FGF-induced cell signaling The Journal of biological chemistry
Jastrebova N, Vanwildemeersch M, Lindahl U, Spillmann D
2010 Aug 27;285(35):26842-26851. doi: 10.1074/jbc.M109.093542. Epub 2010 Jun 24.
-
More
Heparan sulfates (HSs) modulate various developmental and homeostatic processes by binding to protein ligands. We have evaluated the structural characteristics of porcine HS in cellular signaling induced by basic fibroblast growth factor (FGF2), using CHO745 cells devoid of endogenous glycosaminoglycans as target. Markedly enhanced stimulation of cell signaling, measured as phosphorylation of ERK1/2 and protein kinase B, was only observed with the shortest HS chains isolated from liver, whereas the longer chains from either liver or intestine essentially prolonged duration of signals induced by FGF2 in the absence of polysaccharide. Structural analysis showed that contiguous sulfated domains were most abundant in the shortest HS chains and were more heavily sulfated in HS from liver than in HS from intestine. Moreover, the shortest chains from either source entered into ternary complexes with FGF2 and FGF receptor-1c more efficiently than the corresponding longer chains. In addition to authentic HSs, decasaccharide libraries generated by chemo-enzymatic modification of heparin were probed for effect on FGF2 signaling. Only the most highly sulfated decamers, previously found most efficient in ternary complex formation (Jastrebova, N., Vanwildemeersch, M., Rapraeger, A. C., Giménez-Gallego, G., Lindahl, U., and Spillmann, D. (2006) J. Biol. Chem. 281, 26884-26892), promoted FGF2 cellular signaling as efficiently as short HS chains from liver. Together these results suggest that the effects of HS on FGF2 signaling are determined by both the structure of the highly sulfated domains and by the organization/availability of such domains within the HS chain. These findings underpin the need for regulation of HS biosynthesis in relation to control of growth factor-induced signaling pathways.
PMID:20576609 | PMC:PMC2930683 | DOI:10.1074/jbc.M109.093542
View details for PubMedID 20576609
-
More
-
Interaction of syndecan and alpha6beta4 integrin cytoplasmic domains: regulation of ErbB2-mediated integrin activation The Journal of biological chemistry
Wang H, Leavitt L, Ramaswamy R, Rapraeger AC
2010 Apr 30;285(18):13569-79. doi: 10.1074/jbc.M110.102137. Epub 2010 Feb 24.
-
More
The alpha6beta4 integrin is a laminin 332 (LN332) receptor central to the formation of hemidesmosomes in epithelial layers. However, the integrin becomes phosphorylated by keratinocytes responding to epidermal growth factor in skin wounds or by squamous cell carcinomas that overexpress/hyperactivate the tyrosine kinase ErbB2, epidermal growth factor receptor, or c-Met. We show here that the beta4-dependent signaling in A431 human squamous carcinoma cells is dependent on the syndecan family of matrix receptors. Yeast two-hybrid analysis identifies an interaction within the distal third (amino acids 1473-1752) of the beta4 cytoplasmic domain and the conserved C2 region of the syndecan cytoplasmic domain. Via its C2 region, Sdc1 forms a complex with the alpha6beta4 integrin along with the receptor tyrosine kinase ErbB2 and the cytoplasmic kinase Fyn in A431 cells. Engagement of LN332 or clustering of the alpha6beta4 integrin with integrin-specific antibodies causes phosphorylation of ErbB2, Fyn, and the beta4 subunit as well as activation of phosphatidylinositol 3-kinase and Akt and their assimilation into this complex. This leads to phosphatidylinositol 3-kinase-dependent cell spreading and Akt-dependent protection from apoptosis. This is disrupted by RNA interference silencing of Sdc1 but can be rescued by mouse Sdc1 or Sdc4 but not by syndecan mutants lacking their C-terminal C2 region. This disruption does not prevent the phosphorylation of ErbB2 or Fyn but blocks the Fyn-mediated phosphorylation of the beta4 tail. We propose that syndecans engage the distal region of the beta4 cytoplasmic domain and bring it to the plasma membrane, where it can be acted upon by Src family kinases.
PMID:20181947 | PMC:PMC2859518 | DOI:10.1074/jbc.M110.102137
View details for PubMedID 20181947
-
More
-
Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis Blood
Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD
2010 Mar 25;115(12):2449-57. doi: 10.1182/blood-2009-07-234757. Epub 2010 Jan 22.
-
More
Heparanase enhances shedding of syndecan-1 (CD138), and high levels of heparanase and shed syndecan-1 in the tumor microenvironment are associated with elevated angiogenesis and poor prognosis in myeloma and other cancers. To explore how the heparanase/syndecan-1 axis regulates angiogenesis, we used myeloma cells expressing either high or low levels of heparanase and examined their impact on endothelial cell invasion and angiogenesis. Medium conditioned by heparanase-high cells significantly stimulated endothelial invasion in vitro compared with medium from heparanase-low cells. The stimulatory activity was traced to elevated levels of vascular endothelial growth factor (VEGF) and syndecan-1 in the medium. We discovered that the heparan sulfate chains of syndecan-1 captured VEGF and also attached the syndecan-1/VEGF complex to the extracellular matrix where it then stimulated endothelial invasion. In addition to its heparan sulfate chains, the core protein of syndecan-1 was also required because endothelial invasion was blocked by addition of synstatin, a peptide mimic of the integrin activating region present on the syndecan-1 core protein. These results reveal a novel mechanistic pathway driven by heparanase expression in myeloma cells whereby elevated levels of VEGF and shed syndecan-1 form matrix-anchored complexes that together activate integrin and VEGF receptors on adjacent endothelial cells thereby stimulating tumor angiogenesis.
PMID:20097882 | PMC:PMC2845901 | DOI:10.1182/blood-2009-07-234757
View details for PubMedID 20097882
-
More
-
Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor The Journal of experimental medicine
Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC
2009 Mar 16;206(3):691-705. doi: 10.1084/jem.20081278. Epub 2009 Mar 2.
-
More
Syndecan-1 (Sdc1) is a matrix receptor shown to associate via its extracellular domain with the alpha(v)beta(3) and alpha(v)beta(5) integrins, potentially regulating cell adhesion, spreading, and invasion of cells expressing these integrins. Using Sdc1 deletion mutants expressed in human mammary carcinoma cells, we identified the active site within the Sdc1 core protein and derived a peptide inhibitor called synstatin (SSTN) that disrupts Sdc1's interaction with these integrins. Because the alpha(v)beta(3) and alpha(v)beta(5) integrins are critical in angiogenesis, a process in which a role for Sdc1 has been uncertain, we used human vascular endothelial cells in vitro to show that the Sdc1 regulatory mechanism is also required for integrin activation on these cells. We found Sdc1 expressed in the vascular endothelium during microvessel outgrowth from aortic explants in vitro and in mouse mammary tumors in vivo. Moreover, we show that SSTN blocks angiogenesis in vitro or when delivered systemically in a mouse model of angiogenesis in vivo, and impairs mammary tumor growth in an orthotopic mouse tumor model. Thus, Sdc1 is a critical regulator of these two important integrins during angiogenesis and tumorigenesis, and is inhibited by the novel SSTN peptide.
PMID:19255147 | PMC:PMC2699122 | DOI:10.1084/jem.20081278
View details for PubMedID 19255147
-
More
-
Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin The Journal of cell biology
Ma P, Beck SL, Raab RW, McKown RL, Coffman GL, Utani A, Chirico WJ, Rapraeger AC, Laurie GW
2006 Sep 25;174(7):1097-106. doi: 10.1083/jcb.200511134. Epub 2006 Sep 18.
-
More
Cell surface heparan sulfate (HS) proteoglycans are carbohydrate-rich regulators of cell migratory, mitogenic, secretory, and inflammatory activity that bind and present soluble heparin-binding growth factors (e.g., fibroblast growth factor, Wnt, Hh, transforming growth factor beta, amphiregulin, and hepatocyte growth factor) to their respective signaling receptors. We demonstrate that the deglycanated core protein of syndecan-1 (SDC1) and not HS chains nor SDC2 or -4, appears to target the epithelial selective prosecretory mitogen lacritin. An important and novel step in this mechanism is that binding necessitates prior partial or complete removal of HS chains by endogenous heparanase. This limits lacritin activity to sites where heparanase appears to predominate, such as sites of exocrine cell migration, secretion, renewal, and inflammation. Binding is mutually specified by lacritin's C-terminal mitogenic domain and SDC1's N terminus. Heparanase modification of the latter transforms a widely expressed HS proteoglycan into a highly selective surface-binding protein. This novel example of cell specification through extracellular modification of an HS proteoglycan has broad implications in development, homeostasis, and disease.
PMID:16982797 | PMC:PMC1666580 | DOI:10.1083/jcb.200511134
View details for PubMedID 16982797
-
More
-
Heparan sulfate-related oligosaccharides in ternary complex formation with fibroblast growth factors 1 and 2 and their receptors The Journal of biological chemistry
Jastrebova N, Vanwildemeersch M, Rapraeger AC, Giménez-Gallego G, Lindahl U, Spillmann D
2006 Sep 15;281(37):26884-92. doi: 10.1074/jbc.M600806200. Epub 2006 Jun 28.
-
More
Biosynthesis of heparan sulfate (HS) is strictly regulated to yield products with cell/tissue-specific composition. Interactions between HS and a variety of proteins, including growth factors and morphogens, are essential for embryonic development and for homeostasis in the adult. Fibroblast growth factors (FGFs) and their various receptors (FRs) form ternary complexes with HS, as required for receptor signaling. Libraries of HS-related, radiolabeled oligosaccharides were generated by chemo-enzymatic modification of heparin and tested for affinity to immobilized FR ectodomains in the presence of FGF1 or FGF2. Experiments were designed to enable assessment of N-sulfated 8- and 10-mers with defined numbers of iduronic acid 2-O-sulfate and glucosamine 6-O-sulfate groups. FGF1 and FGF2 were found to require similar oligosaccharides in complex formation with FR1c-3c, FGF2 affording somewhat more efficient oligosaccharide recruitment than FGF1. FR4, contrary to FR1c-3c, bound oligosaccharides at physiological ionic conditions even in the absence of FGFs, and this interaction was further promoted by FGF1 but not by FGF2. In all systems studied, the stability of FGF-oligosaccharide-FR complexes correlated with the overall level of saccharide O-sulfation rather than on the precise distribution of sulfate groups.
PMID:16807244 | DOI:10.1074/jbc.M600806200
View details for PubMedID 16807244
-
More
-
Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts Journal of cell science
McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC
2006 Jun 15;119(Pt 12):2445-56. doi: 10.1242/jcs.02970. Epub 2006 May 23.
-
More
B82L mouse fibroblasts respond to fibronectin or vitronectin via a syndecan-1-mediated activation of the alphavbeta5 integrin. Cells attached to syndecan-1-specific antibody display only filopodial extension. However, the syndecan-anchored cells extend lamellipodia when the antibody-substratum is supplemented with serum, or low concentrations of adsorbed vitronectin or fibronectin, that are not sufficient to activate the integrin when plated alone. Integrin activation is blocked by treatment with (Arg-Gly-Asp)-containing peptides and function-blocking antibodies that target alphav integrins, as well as by siRNA-mediated silencing of beta5 integrin expression. In addition, alphavbeta5-mediated cell attachment and spreading on high concentrations of vitronectin is blocked by competition with recombinant syndecan-1 ectodomain core protein and by downregulation of mouse syndecan-1 expression by mouse-specific siRNA. Taking advantage of the species-specificity of the siRNA, rescue experiments in which human syndecan-1 constructs are expressed trace the activation site to the syndecan-1 ectodomain. Moreover, both full-length mouse and human syndecan-1 co-immunoprecipitate with the beta5 integrin subunit, but fail to do so if the syndecan is displaced by competition with soluble, recombinant syndecan-1 ectodomain. These results suggest that the ectodomain of the syndecan-1 core protein contains an active site that assembles into a complex with the alphavbeta5 integrin and regulates alphavbeta5 integrin activity.
PMID:16720645 | DOI:10.1242/jcs.02970
View details for PubMedID 16720645
-
More
-
HSulf-1 and HSulf-2 are potent inhibitors of myeloma tumor growth in vivo The Journal of biological chemistry
Dai Y, Yang Y, MacLeod V, Yue X, Rapraeger AC, Shriver Z, Venkataraman G, Sasisekharan R, Sanderson RD
2005 Dec 2;280(48):40066-73. doi: 10.1074/jbc.M508136200. Epub 2005 Sep 27.
-
More
To participate as co-receptor in growth factor signaling, heparan sulfate must have specific structural features. Recent studies show that when the levels of 6-O-sulfation of heparan sulfate are diminished by the activity of extracellular heparan sulfate 6-O-endosulfatases (Sulfs), fibroblast growth factor 2-, heparin binding epidermal growth factor-, and hepatocyte growth factor-mediated signaling are attenuated. This represents a novel mechanism for regulating cell growth, particularly within the tumor microenvironment where the Sulfs are known to be misregulated. To directly test the role of Sulfs in tumor growth control in vivo, a human myeloma cell line was transfected with cDNAs encoding either of the two known human endosulfatases, HSulf-1 or HSulf-2. When implanted into severe combined immunodeficient (SCID) mice, the growth of these tumors was dramatically reduced on the order of 5- to 10-fold as compared with controls. In addition to an inhibition of tumor growth, these studies revealed the following. (i) HSulf-1 and HSulf-2 have similar functions in vivo. (ii) The extracellular activity of Sulfs is restricted to the local tumor cell surface. (iii) The Sulfs promote a marked increase in extracellular matrix deposition within tumors that may, along with attenuated growth factor signaling, contribute to the reduction in tumor growth. These findings demonstrate that dynamic regulation of heparan sulfate structure by Sulfs present within the tumor microenvironment can have a dramatic impact on the growth and progression of malignant cells in vivo.
PMID:16192265 | DOI:10.1074/jbc.M508136200
View details for PubMedID 16192265
-
More
-
Regulation of fibroblast growth factor-2 activity by human ovarian cancer tumor endothelium Clinical cancer research : an official journal of the American Association for Cancer Research
Whitworth MK, Backen AC, Clamp AR, Wilson G, McVey R, Friedl A, Rapraeger AC, David G, McGown A, Slade RJ, Gallagher JT, Jayson GC
2005 Jun 15;11(12):4282-8. doi: 10.1158/1078-0432.CCR-04-1386.
-
More
Fibroblast growth factor-2 (FGF-2) is a potent angiogenic cytokine that is dependent on heparan sulfate for its biological activity. We have investigated the relationship among heparan sulfate, FGF-2, and the signal-transducing receptors in human, advanced-stage, serous ovarian adenocarcinoma. Using a unique molecular probe, FR1c-Ap, which consisted of a soluble FGF receptor 1 isoform IIIc covalently linked to an alkaline phosphatase moiety, the distribution of heparan sulfate that had the ability to support the formation of a heparan sulfate/FGF-2/FGFR1 isoform IIIc alkaline phosphatase heparan sulfate construct complex was determined. This may be taken as a surrogate marker for the distribution of biologically active heparan sulfate and was distributed predominantly in endothelial cells and stroma but was absent from adenocarcinoma cells. In situ hybridization revealed the expression of FGFR1 mRNA in the endothelium and reverse transcription-PCR confirmed the presence of FGFR1 isoform IIIc but not isoform IIIb. The presence of FGF-2 around tumor endothelium was detected through immunohistochemistry. Double-staining techniques showed that heparan sulfate was found predominantly at the basal aspect of the endothelium and suggested that syndecan-3 might function as one of the proteoglycans involved in FGF-2 signaling in the endothelium. The data suggest that the entire extracellular signaling apparatus, consisting of FGF-2, biologically active heparan sulfate, and FGFRs capable of responding to FGF-2, is present in ovarian cancer endothelium, thereby highlighting the cytokine and its cognate receptor as potential targets for the antiangiogenic treatment of this disease.
PMID:15958608 | DOI:10.1158/1078-0432.CCR-04-1386
View details for PubMedID 15958608
-
More
-
The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells The Journal of cell biology
Beauvais DM, Burbach BJ, Rapraeger AC
2004 Oct 11;167(1):171-81. doi: 10.1083/jcb.200404171.
-
More
The alpha(v)beta(3) integrin participates in cell morphogenesis, growth factor signaling, and cell survival. Activation of the integrin is central to these processes and is influenced by specific ECM components, which engage both integrins and syndecans. This paper demonstrates that the alpha(v)beta(3) integrin and syndecan-1 (S1) are functionally coupled. The integrin is dependent on the syndecan to become activated and to mediate signals required for MDA-MB-231 and MDA-MB-435 human mammary carcinoma cell spreading on vitronectin or S1-specific antibody. Coupling of the syndecan to alpha(v)beta(3) requires the S1 ectodomain (ED), as ectopic expression of glycosylphosphatidylinositol-linked S1ED enhances alpha(v)beta(3) recognition of vitronectin; and treatments that target this domain, including competition with recombinant S1ED protein or anti-S1ED antibodies, mutation of the S1ED, or down-regulation of S1 expression by small-interfering RNAs, disrupt alpha(v)beta(3)-dependent cell spreading and migration. Thus, S1 is likely to be a critical regulator of many cellular behaviors that depend on activated alpha(v)beta(3) integrins.
PMID:15479743 | PMC:PMC2172512 | DOI:10.1083/jcb.200404171
View details for PubMedID 15479743
-
More
-
Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells Experimental cell research
Burbach BJ, Ji Y, Rapraeger AC
2004 Oct 15;300(1):234-47. doi: 10.1016/j.yexcr.2004.07.001.
-
More
Syndecan-1 was overexpressed in T47D, MCF-7, or Hs578t human breast carcinoma cell lines, mimicking overexpression observed in carcinomas in vivo. Overexpression of syndecan-1, or its ectodomain alone fused to a glycosylphosphatidylinositol anchor (GPI-mS1ED), promotes cell rounding in 2D culture. Deletions within the syndecan-1 ectodomain (S1ED) implicate an active site within the core protein between the glycosaminoglycan attachment region and the transmembrane domain. Polyclonal antibodies directed against the ectodomain, or treatment with the tyrosine kinase inhibitor genistein, block activity and revert GPI-mS1ED overexpressing cells to a normal morphology. Extracellular matrix (ECM)-dependent signaling appears to be targeted, as GPI-mS1ED cells attach and spread similarly to control cells in response to E-cadherin engagement, but fail to spread on integrin-dependent ligands. However, integrin-dependent cell attachment, and integrin activation and subsequent FAK phosphorylation are unaffected, suggesting that the syndecan regulates the integration of signaling following matrix adhesion. In 3D culture, where syndecan-1 may have a more critical role in cell behavior, the disrupted signaling leads to poorly cohesive, invasive colonies. Thus, altered matrix-dependent signaling due to increased levels of cell surface syndecan-1 may lead to epithelial cell invasion during early stages of tumorigenesis.
PMID:15383330 | DOI:10.1016/j.yexcr.2004.07.001
View details for PubMedID 15383330
-
More
-
Cell-surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin The Journal of biological chemistry
Jasuja R, Allen BL, Pappano WN, Rapraeger AC, Greenspan DS
2004 Dec 3;279(49):51289-97. doi: 10.1074/jbc.M408129200. Epub 2004 Sep 20.
-
More
Signaling by bone morphogenetic proteins (BMPs) plays a central role in early embryonic patterning, organogenesis, and homeostasis in a broad range of species. Chordin, an extracellular antagonist of BMP signaling, is thought to readily diffuse in tissues, thus forming gradients of BMP inhibition that result in reciprocal gradients of BMP signaling. The latter determine cell fates along the embryonic dorsoventral axis. The secreted protein Twisted Gastrulation (TSG) is thought to help shape BMP signaling gradients by acting as a cofactor that enhances Chordin inhibition of BMP signaling. Here, we demonstrate that mammalian Chordin binds heparin with an affinity similar to that of factors known to functionally interact with heparan sulfate proteoglycans (HSPGs) in tissues. We further demonstrate that Chordin binding in mouse embryonic tissues was dependent upon its interaction with cell-surface HSPGs and that Chordin bound to cell-surface HSPGs (e.g. syndecans), but not to basement membranes containing the HSPG perlecan. Surprisingly, mammalian TSG did not bind heparin unless prebound to Chordin and/or BMP-4, although Drosophila TSG has been reported to bind heparin on its own. Results are also presented that indicate that Chordin-HSPG interactions strongly potentiate the antagonism of BMP signaling by Chordin and are necessary for the retention and uptake of Chordin by cells. These data and others regarding Chordin diffusion have implications for the paradigm of how Chordin is thought to regulate BMP signaling in the extracellular space and how gradients of BMP signaling are formed.
PMID:15381701 | DOI:10.1074/jbc.M408129200
View details for PubMedID 15381701
-
More
-
Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration Genes & development
Cornelison DW, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB
2004 Sep 15;18(18):2231-6. doi: 10.1101/gad.1214204.
-
More
Syndecan-3 and syndecan-4 function as coreceptors for tyrosine kinases and in cell adhesion. Syndecan-3(-/-) mice exhibit a novel form of muscular dystrophy characterized by impaired locomotion, fibrosis, and hyperplasia of myonuclei and satellite cells. Explanted syndecan-3(-/-) satellite cells mislocalize MyoD, differentiate aberrantly, and exhibit a general increase in overall tyrosine phosphorylation. Following induced regeneration, the hyperplastic phenotype is recapitulated. While there are fewer apparent defects in syndecan-4(-/-) muscle, explanted satellite cells are deficient in activation, proliferation, MyoD expression, myotube fusion, and differentiation. Further, syndecan-4(-/-) satellite cells fail to reconstitute damaged muscle, suggesting a unique requirement for syndecan-4 in satellite cell function.
PMID:15371336 | PMC:PMC517515 | DOI:10.1101/gad.1214204
View details for PubMedID 15371336
-
More
-
A polymer scaffold for protein oligomerization Journal of the American Chemical Society
Griffith BR, Allen BL, Rapraeger AC, Kiessling LL
2004 Feb 18;126(6):1608-9. doi: 10.1021/ja037646m.
-
More
We report the design and synthesis of well-defined polymers for the noncovalent oligomerization of proteins. The reported scaffolds, which were generated by atom-transfer radical polymerization (ATRP), take advantage of the well-characterized interaction between a Ni2+ complex and an oligohistidine sequence (His tag). Thus, our polymers are designed to facilitate the oligomerization of any protein possessing a His tag. We demonstrate that they can oligomerize fibroblast growth factor-8b (FGF-8b) and promote FGF-8b-mediated cell proliferation in the absence of heparin.
PMID:14871072 | DOI:10.1021/ja037646m
View details for PubMedID 14871072
-
More
-
Syndecans in tumor cell adhesion and signaling Reproductive biology and endocrinology : RB&E
Beauvais DM, Rapraeger AC
2004 Jan 7;2:3. doi: 10.1186/1477-7827-2-3.
-
More
Anchorage of cells to "heparin"--binding domains that are prevalent in extracellular matrix (ECM) components is thought to occur primarily through the syndecans, a four-member family of transmembrane heparan sulfate proteoglycans that communicate environmental cues from the ECM to the cytoskeleton and the signaling apparatus of the cell. Known activities of the syndecans trace to their highly conserved cytoplasmic domains and to their heparan sulfate chains, which can serve to regulate the signaling of growth factors and morphogens. However, several emerging studies point to critical roles for the syndecans' extracellular protein domains in tumor cell behavior to include cell adhesion and invasion. Although the mechanisms of these activities remain largely unknown, one possibility involves "co-receptor" interactions with integrins that may regulate integrin function and the cell adhesion-signaling phenotype. Thus, alterations in syndecan expression, leading to either overexpression or loss of expression, both of which take place in tumor cells, may have dramatic effects on tumor cell invasion.
PMID:14711376 | PMC:PMC320497 | DOI:10.1186/1477-7827-2-3
View details for PubMedID 14711376
-
More
-
Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly The Journal of cell biology
Allen BL, Rapraeger AC
2003 Nov 10;163(3):637-48. doi: 10.1083/jcb.200307053.
-
More
Heparan sulfate (HS) interacts with diverse growth factors, including Wnt, Hh, BMP, VEGF, EGF, and FGF family members, and is a necessary component for their signaling. These proteins regulate multiple cellular processes that are critical during development. However, a major question is whether developmental changes occur in HS that regulate the activity of these factors. Using a ligand and carbohydrate engagement assay, and focusing on FGF1 and FGF8b interactions with FGF receptor (FR)2c and FR3c, this paper reveals global changes in HS expression in mouse embryos during development that regulate FGF and FR complex assembly. Furthermore, distinct HS requirements are identified for both complex formation and signaling for each FGF and FR pair. Overall, these results suggest that changes in HS act as critical temporal regulators of growth factor and morphogen signaling during embryogenesis.
PMID:14610064 | PMC:PMC2173664 | DOI:10.1083/jcb.200307053
View details for PubMedID 14610064
-
More
-
Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A Journal of cellular biochemistry
Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, Sage EH
2003 Oct 1;90(2):408-23. doi: 10.1002/jcb.10645.
-
More
The role of the matricellular protein SPARC (secreted protein, acidic and rich in cysteine) in modulation of vascular cell proliferation is believed to be mediated, in part, by its ability to regulate the activity of certain growth factors through direct binding. In this study, we demonstrate that SPARC does not bind to basic fibroblast growth factor (bFGF/FGF-2) or interfere with complex formation between FGF-2 and its high-affinity FGF receptor-1 (FGFR1), yet both native SPARC and a peptide derived from the C-terminal high-affinity Ca(2+)-binding region of protein significantly inhibit ligand-induced autophosphorylation of FGFR1 (>80%), activation of mitogen-activated protein kinases (MAPKs) (>75%), and DNA synthesis in human microvascular endothelial cells (HMVEC) stimulated by FGF-2 (>80%). We also report that in the presence of FGF-2, a factor which otherwise stimulates myoblast proliferation and the repression of terminal differentiation, both native SPARC and the Ca(2+)-binding SPARC peptide significantly promote (>60%) the differentiation of the MM14 murine myoblast cell line that expresses FGFR1 almost exclusively. Moreover, using heparan sulfate proteoglycan (HSPG)-deficient myeloid cells and porcine aortic endothelial cells (PAECs) expressing chimeric FGFR1, we show that antagonism of FGFR1-mediated DNA synthesis and MAPK activation by SPARC does not require the presence of cell-surface, low-affinity FGF-2 receptors, but can be mediated by an intracellular mechanism that is independent of an interaction with the extracellular ligand-binding domain of FGFR1. We also report that the inhibitory effect of SPARC on DNA synthesis and MAPK activation in endothelial cells is mediated in part (>50%) by activation of protein kinase A (PKA), a known regulator of Raf-MAPK pathway. SPARC thus modulates the mitogenic effect of FGF-2 downstream from FGFR1 by selective regulation of the MAPK signaling cascade.
PMID:14505356 | DOI:10.1002/jcb.10645
View details for PubMedID 14505356
-
More
-
Syndecan-1 transmembrane and extracellular domains have unique and distinct roles in cell spreading The Journal of biological chemistry
McQuade KJ, Rapraeger AC
2003 Nov 21;278(47):46607-15. doi: 10.1074/jbc.M304775200. Epub 2003 Sep 14.
-
More
Raji cells expressing syndecan-1 (Raji-S1) adhere and spread when plated on heparan sulfate-binding extracellular matrix ligands or monoclonal antibody 281.2, an antibody directed against the syndecan-1 extracellular domain. Cells plated on monoclonal antibody 281.2 initially extend a broad lamellipodium, a response accompanied by membrane ruffling at the cell margin. Membrane ruffling then becomes polarized, leading to an elongated cell morphology. Previous work demonstrated that the syndecan-1 cytoplasmic domain is not required for these activities, suggesting important roles for the syndecan-1 transmembrane and/or extracellular domains in the assembly of a signaling complex necessary for spreading. Work described here demonstrates that truncation of the syndecan-1 extracellular domain does not affect the initial lamellipodial extension in the Raji-S1 cells but does inhibit the active membrane ruffling that is necessary for cell polarization. Replacement of the entire syndecan-1 transmembrane domain with leucine residues completely blocks the cell spreading. These data demonstrate that the syndecan-1 transmembrane and extracellular domains have important but distinct roles in Raji-S1 cell spreading; the extracellular domain mediates an interaction that is necessary for dynamic cytoskeletal rearrangements whereas an interaction of the transmembrane domain is required for the initial spreading response.
PMID:12975379 | DOI:10.1074/jbc.M304775200
View details for PubMedID 12975379
-
More
-
Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells Matrix biology : journal of the International Society for Matrix Biology
Burbach BJ, Friedl A, Mundhenke C, Rapraeger AC
2003 Apr;22(2):163-77. doi: 10.1016/s0945-053x(03)00009-x.
-
More
Expression patterns of syndecan-1, the cell surface heparan sulfate proteoglycan (HSPG) predominant on epithelial cells, were analyzed in tissue samples from 30 infiltrating human breast carcinomas and in 9 human breast carcinoma cell lines. Immunohistochemical staining demonstrates that while a subset of the breast carcinomas lose syndecan-1, this proteoglycan is expressed or overexpressed in a majority of the cases. Interestingly, cells in poor grade tumors contain intracellular syndecan-1, an observation that has not been previously described and was thus further investigated. Examination of cultured breast carcinoma cell lines indicates that they also display the phenotype of the syndecan-1 positive tumors and thereby provide a model system for analysis of intracellular syndecan-1. All cell lines examined express syndecan-1, and poorly differentiated lines such as BT549 cells internalize the proteoglycan from the cell surface where it accumulates as intact HSPG in intracellular vesicles. Colocalization studies using fluorescent markers identify these to be lysosomes. This finding is unexpected, as the accepted mechanism for degradation of syndecan HSPG following endocytosis is fragmentation of the protein core and glycosaminoglycan chains in endosomes, followed by delivery of the fragments to lysosomes. Lysosomal inactivation using ammonium chloride demonstrates that well-differentiated lines such as T47D and MCF-7 cells, which maintain the majority of syndecan-1 on their cell surfaces, also target intact constitutively endocytosed syndecan-1 to lysosomes. Taken together, these results suggest that mammary epithelial cells utilize a previously uncharacterized mechanism for syndecan-1 catabolism. In this pathway the proteoglycan remains intact as it passes through the endosomal system, prior to arriving at its site of intracellular degradation in lysosomes.
PMID:12782143 | DOI:10.1016/s0945-053x(03)00009-x
View details for PubMedID 12782143
-
More
-
Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells Experimental cell research
Beauvais DM, Rapraeger AC
2003 Jun 10;286(2):219-32. doi: 10.1016/s0014-4827(03)00126-5.
-
More
Syndecans are cell surface heparan sulfate proteoglycans with regulatory roles in cell adhesion, proliferation, and differentiation [Annu. Rev. Biochem. 68 (1999) 729]. While the syndecan heparan sulfate chains are essential for matrix binding, less is known about the signaling role of their core proteins. To mimic syndecan-specific adhesion, MDA-MB-231 mammary carcinoma cells were plated on antibodies against syndecan-4 or syndecan-1. While cells adherent via syndecan-4 spread, cells adherent via syndecan-1 do not. However, cells adherent via syndecan-1 can be induced to spread by Mn(2+), suggesting that activation of a beta(1) or beta(3) integrin partner is required. Surprisingly, pretreatment of cells with a function-activating beta(1) antibody does not induce spreading, whereas function-blocking beta(1) integrin antibodies do, suggesting involvement of a beta(1)-to-beta(3) integrin cross-talk. Indeed, blockade of beta(1) integrin activation induces alpha(v)beta(3) integrin activation detectable by soluble fibrinogen binding. Spreading in response to syndecan-1 is independent of integrin-ligand binding. Furthermore, competition with soluble murine syndecan-1 ectodomain, which does not disrupt cell adhesion, nonetheless blocks the spreading mechanism. These data suggest that the ectodomain of the syndecan-1 core protein directly participates in the formation of a signaling complex that signals in cooperation with alpha(v)beta(3) integrins; signaling via this complex is negatively regulated by beta(1) integrins.
PMID:12749851 | DOI:10.1016/s0014-4827(03)00126-5
View details for PubMedID 12749851
-
More
-
Heparan sulfate-growth factor interactions Methods in cell biology
Rapraeger AC
2002;69:83-109. doi: 10.1016/s0091-679x(02)69009-0.
-
Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration Developmental biology
Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB
2001 Nov 1;239(1):79-94. doi: 10.1006/dbio.2001.0416.
-
More
Myogenesis in the embryo and the adult mammal consists of a highly organized and regulated sequence of cellular processes to form or repair muscle tissue that include cell proliferation, migration, and differentiation. Data from cell culture and in vivo experiments implicate both FGFs and HGF as critical regulators of these processes. Both factors require heparan sulfate glycosaminoglycans for signaling from their respective receptors. Since syndecans, a family of cell-surface transmembrane heparan sulfate proteoglycans (HSPGs) are implicated in FGF signaling and skeletal muscle differentiation, we examined the expression of syndecans 1-4 in embryonic, fetal, postnatal, and adult muscle tissue, as well as on primary adult muscle fiber cultures. We show that syndecan-1, -3, and -4 are expressed in developing skeletal muscle tissue and that syndecan-3 and -4 expression is highly restricted in adult skeletal muscle to cells retaining myogenic capacity. These two HSPGs appear to be expressed exclusively and universally on quiescent adult satellite cells in adult skeletal muscle tissue, suggesting a role for HSPGs in satellite cell maintenance or activation. Once activated, all satellite cells maintain expression of syndecan-3 and syndecan-4 for at least 96 h, also implicating these HSPGs in muscle regeneration. Inhibition of HSPG sulfation by treatment of intact myofibers with chlorate results in delayed proliferation and altered MyoD expression, demonstrating that heparan sulfate is required for proper progression of the early satellite cell myogenic program. These data suggest that, in addition to providing potentially useful new markers for satellite cells, syndecan-3 and syndecan-4 may play important regulatory roles in satellite cell maintenance, activation, proliferation, and differentiation during skeletal muscle regeneration.
PMID:11784020 | DOI:10.1006/dbio.2001.0416
View details for PubMedID 11784020
-
More
-
Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition The Journal of cell biology
Allen BL, Filla MS, Rapraeger AC
2001 Nov 26;155(5):845-58. doi: 10.1083/jcb.200106075. Epub 2001 Nov 26.
-
More
FGF signaling uses receptor tyrosine kinases that form high-affinity complexes with FGFs and heparan sulfate (HS) proteoglycans at the cell surface. It is hypothesized that assembly of these complexes requires simultaneous recognition of distinct sulfation patterns within the HS chain by FGF and the FGF receptor (FR), suggesting that tissue-specific HS synthesis may regulate FGF signaling. To address this, FGF-2 and FGF-4, and extracellular domain constructs of FR1-IIIc (FR1c) and FR2-IIIc (FR2c), were used to probe for tissue-specific HS in embryonic day 18 mouse embryos. Whereas FGF-2 binds HS ubiquitously, FGF-4 exhibits a restricted pattern, failing to bind HS in the heart and blood vessels and failing to activate signaling in mouse aortic endothelial cells. This suggests that FGF-4 seeks a specific HS sulfation pattern, distinct from that of FGF-2, which is not expressed in most vascular tissues. Additionally, whereas FR2c binds all FGF-4-HS complexes, FR1c fails to bind FGF-4-HS in most tissues, as well as in Raji-S1 cells expressing syndecan-1. Proliferation assays using BaF3 cells expressing either FR1c or FR2c support these results. This suggests that FGF and FR recognition of specific HS sulfation patterns is critical for the activation of FGF signaling, and that synthesis of these patterns is regulated during embryonic development.
PMID:11724824 | PMC:PMC2150861 | DOI:10.1083/jcb.200106075
View details for PubMedID 11724824
-
More
-
Tissue-specific binding by FGF and FGF receptors to endogenous heparan sulfates Methods in molecular biology (Clifton, N.J.)
Friedl A, Filla M, Rapraeger AC
2001;171:535-46. doi: 10.1385/1-59259-209-0:535.
-
Molecular interactions of syndecans during development Seminars in cell & developmental biology
Rapraeger AC
2001 Apr;12(2):107-16. doi: 10.1006/scdb.2000.0239.
-
More
The syndecans, cell surface heparan sulfate proteoglycans (HSPGs), bind numerous ligands via their HS glycosaminoglycan chains. The response to this binding is flavored by the identity of the core protein that bears the HS chains. Each of the syndecan core proteins has a short cytoplasmic domain that binds cytosolic regulatory factors. The syndecans also contain highly conserved transmembrane domain and extracellular domains for which important activities are slowly emerging. These protein domains, which will be the focus of this review, localize the syndecan to sites at the cell surface during development where they collaborate with other receptors to regulate signaling and cytoskeletal organization.
PMID:11292376 | DOI:10.1006/scdb.2000.0239
View details for PubMedID 11292376
-
More
-
Syndecan-1 signals independently of beta1 integrins during Raji cell spreading Experimental cell research
Lebakken CS, McQuade KJ, Rapraeger AC
2000 Sep 15;259(2):315-25. doi: 10.1006/excr.2000.4981.
-
More
Syndecan-1-expressing Raji lymphoid cells (Raji-S1 cells) bind and spread rapidly when attaching to matrix ligands that contain heparan sulfate-binding domains. However, these ligands also contain binding sites for integrins, which are widely known to signal, raising the question of whether the proteoglycan core protein participates in generation of the signal for spreading. To address this question, the spreading of the Raji-S1 cells is examined on ligands specific for either beta1 integrins, known to be present on the Raji cells, or the syndecan-1 core protein. The cells adhere and spread on invasin, a ligand that activates beta1 integrins, the IIICS fragment of fibronectin, which is a specific ligand for the alpha4beta1 integrin, or mAb281.2, an antibody specific for the syndecan-1 core protein. The signaling resulting from adhesion to the syndecan-specific antibody appears integrin independent as (i) the morphology of the cells spreading on the antibody is distinct from spreading initiated by the integrins alone; (ii) spreading on the syndecan or integrin ligands is affected differently by the kinase inhibitors tyrphostin 25, genistein, and staurosporine; and (iii) spreading on the syndecan-specific antibody is not disrupted by blocking beta1 integrin activation with mAb13, a beta1 inhibitory antibody. These data demonstrate that ligation of syndecan-1 initiates intracellular signaling and suggest that this signaling occurs when cells expressing syndecan-1 adhere to matrix ligands containing heparan sulfate-binding domains.
PMID:10964499 | DOI:10.1006/excr.2000.4981
View details for PubMedID 10964499
-
More
-
Syndecan-regulated receptor signaling The Journal of cell biology
Rapraeger AC
2000 May 29;149(5):995-8. doi: 10.1083/jcb.149.5.995.
-
Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ FASEB journal : official publication of the Federation of American Societies for Experimental Biology
Chang Z, Meyer K, Rapraeger AC, Friedl A
2000 Jan;14(1):137-44. doi: 10.1096/fasebj.14.1.137.
-
More
Fibroblast growth factors (FGFs) require heparan sulfate proteoglycans (HSPGs) as cofactors for signaling. The heparan sulfate chains (HS) mediate stable high affinity binding of FGFs to their receptor tyrosine kinases (FR) and may specifically regulate FGF activity. A novel in situ binding assay was developed to examine the ability of HSPGs to promote FGF/FR binding using a soluble FR fusion construct (FR1-AP). This fusion protein probe forms a dimer in solution, simulating the dimerization or oligomerization that is thought to occur at the cell surface physiologically. In frozen sections of human skin, FGF-2 binds to keratinocytes and basement membranes of epidermis and dermal blood vessels. In contrast, in skin preincubated with FGF-2, FR1-AP binds avidly to FGF-2 immobilized on keratinocyte cell surfaces, but fails to bind to basement membranes at the dermo-epidermal junction or dermal microvessels despite the fact that these structures bind large amounts of FGF-2. Apparently, basement membrane and cell surface HSPGs differ in their ability to mediate the assembly of a FGF/FR signaling complex presumably due to structural differences of the heparan sulfate chains.
PMID:10627288 | DOI:10.1096/fasebj.14.1.137
View details for PubMedID 10627288
-
More
-
Tyrosine phosphorylation of syndecan-1 and -4 cytoplasmic domains in adherent B82 fibroblasts The Journal of biological chemistry
Ott VL, Rapraeger AC
1998 Dec 25;273(52):35291-8. doi: 10.1074/jbc.273.52.35291.
-
More
The syndecans, a family of cell surface proteoglycans, have highly conserved cytoplasmic domains that bind proteins containing PDZ domains and co-localize with the actin cytoskeleton. The syndecan cytoplasmic domains contain four conserved tyrosine residues, two of which are located within favorable sequences for phosphorylation. Endogenous tyrosine phosphorylation of syndecans-1 and -4 is detected in adherent B82 fibroblasts. Approximately 1.5% of total syndecan is endogenously phosphorylated, while most, if not all, cell surface syndecan is phosphorylated following treatment with the tyrosine phosphatase inhibitor pervanadate. Syndecan phosphorylation is also detected in Raji-S1 and NMuMG cells, but only following treatment with vanadate or pervanadate, suggesting that endogenous phosphorylation is maintained in an "off" state in these cells. Endogenous syndecan phosphorylation in B82 cells is rapidly blocked by genistein (IC50 < 10 microM) confirming the presence of a constitutively active kinase and a corresponding tyrosine phosphatase. Phosphorylation is also inhibited by herbimycin A (IC50 < 1.0 microM) and staurosporine (IC50 < 1.0 nM), suggesting a role for Src family kinases in regulating syndecan phosphorylation. Together, these data suggest an important role for tyrosine phosphorylation of the syndecan cytoplasmic domains in regulating downstream signaling events in response to cell adhesion and/or growth factor activity.
PMID:9857070 | DOI:10.1074/jbc.273.52.35291
View details for PubMedID 9857070
-
More
-
Molecular interactions of the syndecan core proteins Current opinion in cell biology
Rapraeger AC, Ott VL
1998 Oct;10(5):620-8. doi: 10.1016/s0955-0674(98)80038-0.
-
More
The syndecan family of cell-surface heparan sulfate proteoglycans participate in multiple cell behaviors ranging from growth factor signaling to cell adhesion. Participation in these activities is dependent on specific binding interactions of their heparan sulfate chains and molecular interactions of their core proteins with cytoskeletal and signaling molecules. The highly conserved features of the core proteins have long suggested important functions, which are only now beginning to be understood. Recent advances point to important roles for the extracellular, transmembrane and cytoplasmic domains of the syndecan core proteins in the assembly of these proteoglycans into an intracellular cytoskeletal and signaling apparatus. The proteins display interactions that may be common among the different family members, as well as interactions that provide signaling capabilities that are specific to individual members.
PMID:9818173 | DOI:10.1016/s0955-0674(98)80038-0
View details for PubMedID 9818173
-
More
-
Characterization of the high affinity cell-binding domain in the cell surface proteoglycan syndecan-4 The Journal of biological chemistry
McFall AJ, Rapraeger AC
1998 Oct 23;273(43):28270-6. doi: 10.1074/jbc.273.43.28270.
-
More
The syndecan family of cell surface proteoglycans regulates cell adhesion via their glycosaminoglycan chains and discrete domains of their core proteins. Core protein domains that are variable between syndecan family members may regulate syndecan-specific associations, thereby endowing individual syndecans with unique functions. A syndecan-4-specific domain has been identified in the extracellular syndecan-4 protein. This region mediates cell adhesion when provided as an artificial substratum and is localized within amino acids 56-109 of the recombinant extracellular protein domain of mouse syndecan-4 (mS4ED) (McFall, A. J., and Rapraeger, A. C. (1997) J. Biol. Chem. 272, 12901-12904). To characterize its interaction with the cell surface, radiolabeled ligand binding studies were performed. A single high affinity interaction, with a dissociation constant of 2 x 10(-9) M, was observed between mS4ED and both human and mouse cells. Both chicken S4ED and mS4ED compete for this interaction, although they are only 34% identical within the cell-binding domain sequence. The extracellular protein domains of syndecan-1, -2, and -3, however, fail to compete. The interaction is also observed with native syndecan-4 shed from cell surfaces. Interestingly, the extracellular protein domain of syndecan-1 also mediates cell adhesion, suggesting a similar but discrete interaction for this family member.
PMID:9774449 | DOI:10.1074/jbc.273.43.28270
View details for PubMedID 9774449
-
More
Contact Information
Alan Rapraeger, PhD
1111 Highland Avenue,3053 WIMR
Madison, WI 53705
