I am an associate professor in the Department of Human Oncology with roles in the clinic, research and teaching. In the clinic, my primary focus is patient safety and quality improvement. I serve as the co-chair of our departmental Quality Assurance committee, which oversees many different initiatives related to quality and safety. I also focus on the clinical applications of the TrueBeam radiotherapy platform. This includes preparation of the treatment machine for clinical use, ongoing quality assurance, involvement in the radiosurgery program and implementation of motion management techniques, such as respiratory gating and optical surface imaging.
My research interests closely follow my clinical focus in patient safety and applications of the TrueBeam radiotherapy platform. The Department of Human Oncology has a strong culture of safety. We collect a lot of data to facilitate quality improvement efforts and I use this data to study the effectiveness of different types of interventions. The remainder of my research is focused on high-precision radiotherapy. I study the use of surface imaging for radiosurgery treatments. I also use data science to investigate whether surface imaging can be used to reduce margins in radiotherapy. Finally, I study the use of plastic scintillation detectors for small field dosimetry.
In addition to my clinical and research roles, I teach graduate students, medical students and residents. My goal is to create an environment in which students can develop a more concrete understanding of the fundamental physics that underlie the radiation therapy process while also developing the interpersonal skills needed to succeed in a dynamic, multi-disciplinary clinical environment.
Education
Fellowship, Medical University of South Carolina, Radiation Oncology Physics (2015)
Resident, Medical University of South Carolina, Radiation Oncology Physics (2014)
PhD, University of Wisconsin–Madison, Medical Physics (2012)
MS, University of Wisconsin–Madison, Medical Physics (2008)
BS, University of Wisconsin–Madison, Nuclear Engineering and Engineering Physics (2007)
Academic Appointments
Associate Professor, Human Oncology (2023)
Assistant Professor, Human Oncology (2015)
Selected Honors and Awards
2nd Place–Young Investigator Symposium at AAPM Spring Clinical Meeting (2014)
Graduate Fellowship, American Association of Physicists in Medicine (2008)
Fred W. and Josephine H. Colbeck Scholarship Award (2006)
Max W. Carbon Scholarship in Nuclear Technology (2005)
Kurt F. Wendt Junior Scholarship (2005)
Department of Engineering Physics Nuclear Engineering Scholarship (2004)
Ernest J. Laine Outstanding Sophomore Engineering Student Scholarship (2004)
Boards, Advisory Committees and Professional Organizations
American Association of Physicists in Medicine (2007-pres.)
American Board of Radiology
American Society for Radiation Oncology
Department of Human Oncology Quality Assurance Committee
American College of Radiology (2013-pres.)
Medical Physics Residency Program Oversight Committee (2016-pres.)
AAPM Task Group 341: MPPG 5.b: Commissioning and QA of Treatment Planning Dose Calculations - Megavoltage Photon and Electron Beams
Equipment Donation Sub-Committee of the AAPM/IOMP (2016-pres.)
Radiation Therapy for Rectal Cancer Guideline Task Force
Research Focus
Motion Management, Radiation Measurements, Radiosurgery, Treatment Planning Systems
Dr. Dustin Jacqmin, a medical physicist, focuses primarily on the clinical applications of the TrueBeam radiotherapy platform. This includes preparation of the treatment machine for clinical use, ongoing quality assurance, involvement in the radiosurgery program and implementation of motion management techniques. His research focuses on the TrueBeam radiotherapy platform.
Patient safety and quality improvement, motion management, radiosurgery, small field dosimetry.
During my time at UW, I have worked with my colleagues to implement new treatment techniques and motion management technologies on the TrueBeam radiotherapy platform. The goal of my work is to improve the accuracy of treatment delivery and provide valuable new treatment modalities to my physician partners.
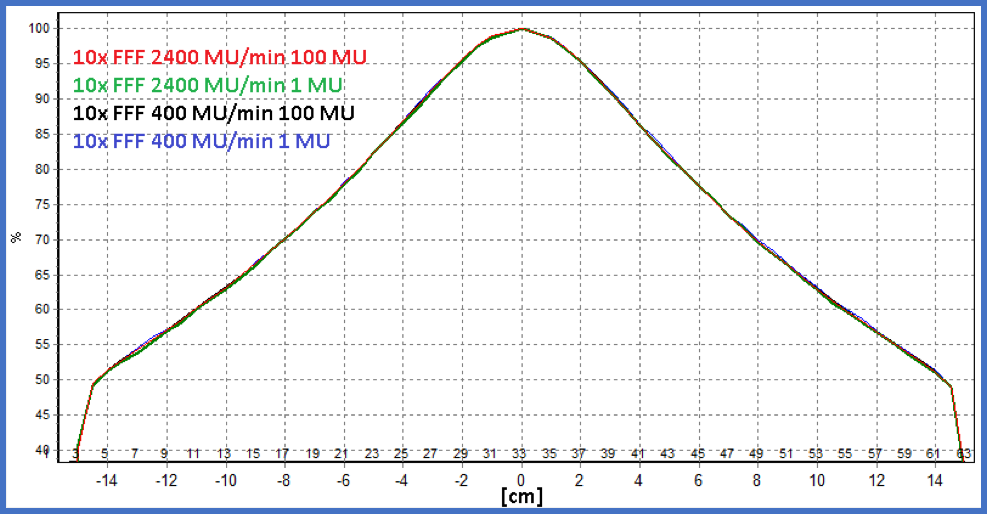
Introduction of 6 MV Flattening Filter Free Photon Beams
The physics team in the Department of Human Oncology has prepared a new treatment modality for fractionated stereotactic radiotherapy (FSRT) and stereotactic body radiotherapy (SBRT) on our Varian TrueBeam linear accelerators. This modality is a different kind of photon beam without a flattening filter—known as “flattening filter free” or FFF beams. Unlike the beams we have used in the past, these beams do not emerge from the machine with a uniform intensity throughout the field. This lack of uniform intensity, considered to be disadvantageous for conventional radiotherapy, is desirable for FSRT and SBRT. Even better: The removal of the flattening filter increases the dose rate up to a factor of four, allowing us to deliver these high-dose-per-fraction treatments in much less time. The physics team has worked hard to ensure that these beams are accurate for the small targets commonly treated with FSRT and SBRT.
Evaluating low-dose-rate performance of the TrueBeam radiotherapy system
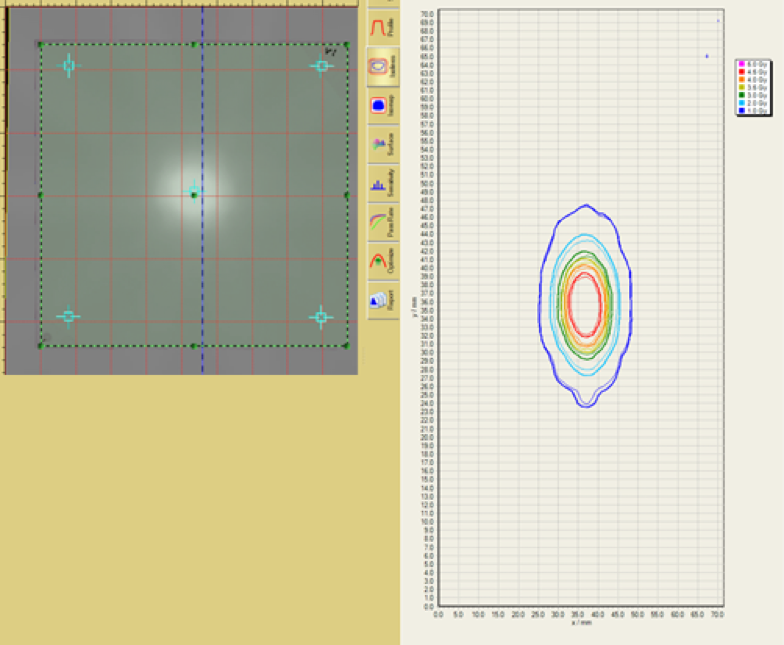
During the commissioning of our new TrueBeam linear accelerator, we investigated the dose rate constancy, MU linearity and profile stability of the TrueBeam radiotherapy delivery system over its full range of available dose rates. The verification of dose-rate constancy, MU linearity and profile stability of the TrueBeam radiotherapy system has clinical relevance for a number of treatment delivery techniques. Dose-rate constancy is important for volumetric modulated arc therapy (VMAT), which modulates the dose rate over the range of available dose rates during arc delivery. Dose-rate constancy at very low dose rates (5-20 MU/min) allows pulsed reduced-dose-rate radiotherapy (PRDR) treatments to be delivered continuously rather than in pulses. Dose-rate constancy at very low dose rates also allows VMAT to be used for PRDR treatments. Finally, the verification of MU linearity down to 2 MU gives greater confidence that small MU segments can be used for intensity modulated radiation therapy (IMRT) and field-in-field delivery.
Evaluating the performance of the optical surface imaging systems
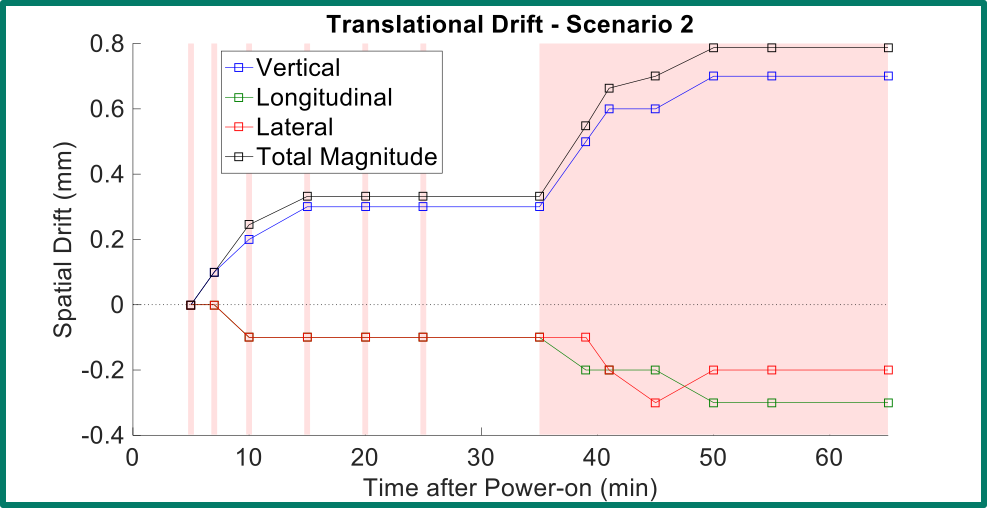
Optical surface imaging systems like AlignRT and the Varian Optical Surface Monitoring System (OSMS) are often used for monitoring patients during frameless stereotactic radiosurgery (SRS). This type of radiotherapy procedure demands sub-millimeter accuracy from the system in order to verify that the patient is within allowable treatment margins during treatment. Optical surface imaging systems are known to exhibit spatial drift during warm-up of the equipment. We investigated the spatial drift of the OSMS system, and our work shows that different warm-up scenarios produce different spatial drift behavior. As a result of this work, we know how to eliminate spatial drift so that it can be used for frameless SRS.
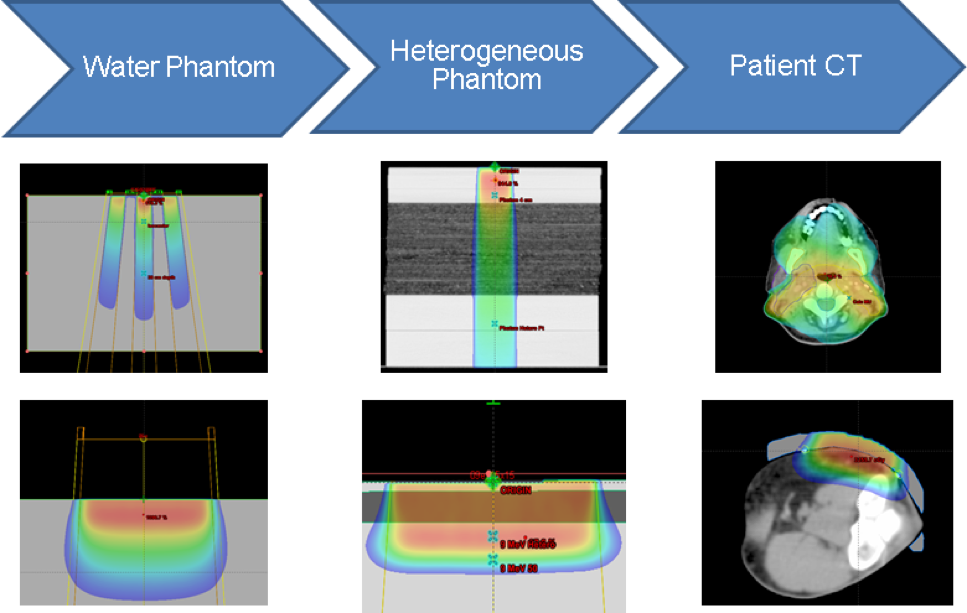
Implementation of the validation testing in MPPG 5.a
The AAPM Medical Physics Practice Guideline (MPPG) 5.a provides concise guidance on the commissioning and QA of beam modeling and dose calculation in radiotherapy treatment planning systems. We worked with physicists at multiple institutions to create a common set of treatment fields and analysis tools to deliver and analyze the validation tests in MPPG 5.a. This included the development of a novel, open-source software tool to compare scanning water tank measurements to 3D DICOM-RT Dose distributions. Dose calculation algorithms in both Pinnacle and Eclipse were tested with MPPG 5.a to validate the modeling of Varian TrueBeam linear accelerators. The validation process resulted in more than 200 water tank scans and more than 50 point measurements per institution, each of which was compared to a dose calculation from the institution’s treatment planning system (TPS). We found the use of MPPG 5.a to be a valuable resource during the commissioning process. We reported our findings in JACMP and have made our tools available to the wider physics community.
-
Software Support Tools for Reirradiation: A Report From Reirradiation Collaborative Group (ReCOG) 2024 International journal of radiation oncology, biology, physics
Matrosic CK, Andrzejewski P, Bergman A, Chng N, Naqa IE, Freislederer P, Hoffmann L, Hope A, Jacqmin D, Kelly C, Kuo L, Lin H, Mayo C, Murrell DH, Ödén J, Svensson S, Thompson S, Osorio EV, West N, Wicha R, Yorke E, Appelt AL, Paradis KC
2025 May 1;122(1):181-185. doi: 10.1016/j.ijrobp.2024.12.023.
-
Ensuring High Quality Treatment Plans with a Plan Quality Review Checklist Practical radiation oncology
Lin M, Olsen L, Kavanaugh JA, Jacqmin D, Lobb E, Yoo S, Berry SL, Pichardo JC, Cardenas CE, Roper J, Kirk M, Bennett S, Cheung JP, Solberg TD, Moore KL, Kim M
2025 Jan-Feb;15(1):e82-e87. doi: 10.1016/j.prro.2024.08.008. Epub 2024 Sep 30.
-
More
Treatment plan quality is a crucial component for a successful outcome of radiation therapy treatments. As the complexity of radiation therapy planning and delivery techniques increases, the role of the medical physicist in assessing treatment plan quality becomes more critical. Integrating plan quality review throughout the treatment planning process allows improvements without delaying treatment or rushing to produce changes at the last minute. In this work, we aim to provide practical check items for physicists to reference when assessing treatment plan quality with a critical eye, asking questions such as "is this the best dose distribution feasible for this patient?," "could we change any planning parameters to improve plan quality?," and "could we change the planning strategy for this particular patient or for future patients?"; and to work with planners and physicians to create a multidisciplinary collaborative culture that achieves the best plan feasible for every patient. We tabulate the features that affect plan quality in each process step and check details for individual items. This report is aimed at medical physicists, planners, radiation oncologists, and other professionals who are involved in treatment planning.
PMID:39357772 | DOI:10.1016/j.prro.2024.08.008
View details for PubMedID 39357772
-
More
-
Beyond Acceptable: The Vital Role of Medical Physicists in Ensuring High-Quality Treatment Plans Practical radiation oncology
Lin M, Olsen L, Kavanaugh JA, Jacqmin D, Lobb E, Yoo S, Berry SL, Pichardo JC, Cardenas CE, Roper J, Kirk M, Cheung JP, Solberg TD, Moore KL, Kim M
2024 Jan-Feb;14(1):6-9. doi: 10.1016/j.prro.2023.08.014.
-
Non-contact scintillator imaging dosimetry for total body irradiation in radiotherapy Physics in medicine and biology
Niver AP, Hammer CG, Culberson WS, Jacqmin D, Pogue BW
2024 Jan 30;69(3):10.1088/1361-6560/ad1a23. doi: 10.1088/1361-6560/ad1a23.
-
More
Objective.The goal of this work was to assess the potential use of non-contact scintillator imaging dosimetry for tracking delivery in total body irradiation (TBI).Approach. Studies were conducted to measure the time-gated light signals caused by radiation exposure to scintillators that were placed on tissue. The purpose was to assess efficacy in conditions common for TBI, such as the large source to surface distance (SSD) commonly used, the reduced dose rate, the inclusion of a plexiglass spoiler, angle of incidence and effects of peripheral patient support structures. Dose validation work was performed on phantoms that mimicked human tissue optical properties and body geometry. For this work, 1.5 cm diameter scintillating disks were developed and affixed to phantoms under various conditions. A time-gated camera synchronized to the linac pulses was used for imaging. Scintillation intensity was quantified in post processing and the values verified with simultaneous thermolumiescent dosimeter (TLD) measurements. Mean scintillation values in each region were compared to TLD measurements to produce dose response curves, and scatter effects from the spoiler and patient bed were quantified.Main results.The dose determined by scintillators placed in TBI conditions agreed with TLD dose determinations to within 2.7%, and did so repeatedly within 1.0% standard deviation variance. A linear fit between scintillator signal and TLD dose was achieved with anR2= 0.996 across several body sites. Scatter from the patient bed resulted in a maximum increase of 19% in dose.Significance.This work suggests that non-contact scintillator imaging dosimetry could be used to verify dose in real time to patients undergoing TBI at the prescribed long SSD and low dose rate. It also has shown that patient transport stretchers can significantly influence surface dose by increasing scatter.
PMID:38171002 | PMC:PMC10915642 | DOI:10.1088/1361-6560/ad1a23
View details for PubMedID 38171002
-
More
-
AAPM MEDICAL PHYSICS PRACTICE GUIDELINE 5.b: Commissioning and QA of treatment planning dose calculations-Megavoltage photon and electron beams Journal of applied clinical medical physics
Geurts MW, Jacqmin DJ, Jones LE, Kry SF, Mihailidis DN, Ohrt JD, Ritter T, Smilowitz JB, Wingreen NE
2022 Sep;23(9):e13641. doi: 10.1002/acm2.13641. Epub 2022 Aug 10.
-
More
The American Association of Physicists in Medicine (AAPM) is a nonprofit professional society whose primary purposes are to advance the science, education, and professional practice of medical physics. The AAPM has more than 8000 members and is the principal organization of medical physicists in the United States. The AAPM will periodically define new practice guidelines for medical physics practice to help advance the science of medical physics and to improve the quality of service to patients throughout the United States. Existing medical physics practice guidelines will be reviewed for the purpose of revision or renewal, as appropriate, on their fifth anniversary or sooner. Each medical physics practice guideline represents a policy statement by the AAPM, has undergone a thorough consensus process in which it has been subjected to extensive review, and requires the approval of the Professional Council. The medical physics practice guidelines recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice guidelines and technical standards by those entities not providing these services is not authorized. The following terms are used in the AAPM practice guidelines: Must and Must Not: Used to indicate that adherence to the recommendation is considered necessary to conform to this practice guideline. While must is the term to be used in the guidelines, if an entity that adopts the guideline has shall as the preferred term, the AAPM considers that must and shall have the same meaning. Should and Should Not: Used to indicate a prudent practice to which exceptions may occasionally be made in appropriate circumstances.
PMID:35950259 | PMC:PMC9512346 | DOI:10.1002/acm2.13641
View details for PubMedID 35950259
-
More
-
Consensus Quality Measures and Dose Constraints for Rectal Cancer From the Veterans Affairs Radiation Oncology Quality Surveillance Program and American Society for Radiation Oncology (ASTRO) Expert Panel Practical radiation oncology
Park J, Venkatesulu BP, Kujundzic K, Katsoulakis E, Solanki AA, Puckett LL, Kapoor R, Chapman CH, Hagan M, Kelly MD, Palta J, Ashman JB, Jacqmin D, Kachnic LA, Minsky BD, Olsen J, Raldow AC, Wo JY, Dawes S, Wilson E, Kudner R, Das P
2022 Sep-Oct;12(5):424-436. doi: 10.1016/j.prro.2022.05.005. Epub 2022 Jun 11.
-
More
PURPOSE: Ensuring high quality, evidence-based radiation therapy for patients with cancer is of the upmost importance. To address this need, the Veterans Affairs (VA) Radiation Oncology Program partnered with the American Society for Radiation Oncology and established the VA Radiation Oncology Quality Surveillance program. As part of this ongoing effort to provide the highest quality of care for patients with rectal cancer, a blue-ribbon panel comprised of rectal cancer experts was formed to develop clinical quality measures.
METHODS AND MATERIALS: The Rectal Cancer Blue Ribbon panel developed quality, surveillance, and aspirational measures for (a) initial consultation and workup, (b) simulation, treatment planning, and treatment, and (c) follow-up. Twenty-two rectal cancer specific measures were developed (19 quality, 1 aspirational, and 2 surveillance). In addition, dose-volume histogram constraints for conventional and hypofractionated radiation therapy were created.
CONCLUSIONS: The quality measures and dose-volume histogram for rectal cancer serves as a guideline to assess the quality of care for patients with rectal cancer receiving radiation therapy. These quality measures will be used for quality surveillance for veterans receiving care both inside and outside the VA system to improve the quality of care for these patients.
PMID:35907764 | DOI:10.1016/j.prro.2022.05.005
View details for PubMedID 35907764
-
More
-
Commissioning an Exradin W2 plastic scintillation detector for clinical use in small radiation fields Journal of applied clinical medical physics
Jacqmin DJ, Miller JR, Barraclough BA, Labby ZE
2022 Aug;23(8):e13728. doi: 10.1002/acm2.13728. Epub 2022 Jul 21.
-
More
PURPOSE: The purpose of this work is to evaluate the Standard Imaging Exradin W2 plastic scintillation detector (W2) for use in the types of fields used for stereotactic radiosurgery.
METHODS: Prior to testing the W2 in small fields, the W2 was evaluated in standard large field conditions to ensure good detector performance. These tests included energy dependence, short-term repeatability, dose-response linearity, angular dependence, temperature dependence, and dose rate dependence. Next, scan settings and calibration of the W2 were optimized to ensure high quality data acquisition. Profiles of small fields shaped by cones and multi-leaf collimator (MLCs) were measured using the W2 and IBA RAZOR diode in a scanning water tank. Output factors for cones (4-17.5 mm) and MLC fields (1, 2, 3 cm) were acquired with both detectors. Finally, the dose at isocenter for seven radiosurgery plans was measured with the W2 detector.
RESULTS: W2 exhibited acceptable warm-up behavior, short-term reproducibility, axial angular dependence, dose-rate linearity, and dose linearity. The detector exhibits a dependence upon energy, polar angle, and temperature. Scanning measurements taken with the W2 and RAZOR were in good agreement, with full-width half-maximum and penumbra widths agreeing to within 0.1 mm. The output factors measured by the W2 and RAZOR exhibited a maximum difference of 1.8%. For the seven point-dose measurements of radiosurgery plans, the W2 agreed well with our treatment planning system with a maximum deviation of 2.2%. The Čerenkov light ratio calibration method did not significantly impact the measurement of relative profiles, output factors, or point dose measurements.
CONCLUSION: The W2 demonstrated dosimetric characteristics that are suitable for radiosurgery field measurements. The detector agreed well with the RAZOR diode for output factors and scanned profiles and showed good agreement with the treatment planning system in measurements of clinical treatment plans.
PMID:35861648 | PMC:PMC9359019 | DOI:10.1002/acm2.13728
View details for PubMedID 35861648
-
More
-
The impact of COVID-19 on a high-volume incident learning system: A retrospective analysis Journal of applied clinical medical physics
Jacqmin DJ, Crosby SM
2022 Jul;23(7):e13653. doi: 10.1002/acm2.13653. Epub 2022 May 26.
-
More
PURPOSE: The purpose of this work was to assess how the coronavirus disease 2019 (COVID-19) pandemic impacted our incident learning system data and communicate the impact of a major exogenous event on radiation oncology clinical practice.
METHODS: Trends in our electronic incident reporting system were analyzed to ascertain the impact of the COVID-19 pandemic, including any direct clinical changes. Incident reports submitted in the 18 months prior to the pandemic (September 14, 2018 to March 13, 2020) and reports submitted during the first 18 months of the pandemic (March 14, 2020 to September 13, 2021) were compared. The incident reports include several data elements that were evaluated for trends between the two time periods, and statistical analysis was performed to compare the proportions of reports.
RESULTS: In the 18 months prior to COVID-19, 192 reports were submitted per 1000 planning tasks (n = 832 total). In the first 18 months of the pandemic, 147 reports per 1000 planning tasks were submitted (n = 601 total), a decrease of 23.4%. Statistical analysis revealed that there were no significant changes among the data elements between the pre- and during COVID-19 time periods. An analysis of the free-text narratives in the reports found that phrases related to pretreatment imaging were common before COVID-19 but not during. Conversely, phrases related to intravenous contrast, consent for computed tomography, and adaptive radiotherapy became common during COVID-19.
CONCLUSIONS: The data elements captured by our incident learning system were stable after the onset of the COVID-19 pandemic, with no statistically significant findings after correction for multiple comparisons. A trend toward fewer reports submitted for low-risk issues was observed. The methods used in the work can be generalized to events with a large-scale impact on the clinic or to monitor an incident learning system to drive future improvement activities.
PMID:35616007 | PMC:PMC9278685 | DOI:10.1002/acm2.13653
View details for PubMedID 35616007
-
More
-
Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline Practical radiation oncology
Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT, Dorth J, Garcia-Aguilar J, Goff D, Jacqmin D, Kelly P, Newman NB, Olsen J, Raldow AC, Ruiz-Garcia E, Stitzenberg KB, Thomas CR, Wu QJ, Das P
2021 Jan-Feb;11(1):13-25. doi: 10.1016/j.prro.2020.08.004. Epub 2020 Oct 21.
-
More
PURPOSE: This guideline reviews the evidence and provides recommendations for the indications and appropriate technique and dose of neoadjuvant radiation therapy (RT) in the treatment of localized rectal cancer.
METHODS: The American Society for Radiation Oncology convened a task force to address 4 key questions focused on the use of RT in preoperative management of operable rectal cancer. These questions included the indications for neoadjuvant RT, identification of appropriate neoadjuvant regimens, indications for consideration of a nonoperative or local excision approach after chemoradiation, and appropriate treatment volumes and techniques. Recommendations were based on a systematic literature review and created using a predefined consensus-building methodology and system for grading evidence quality and recommendation strength.
RESULTS: Neoadjuvant RT is recommended for patients with stage II-III rectal cancer, with either conventional fractionation with concurrent 5-FU or capecitabine or short-course RT. RT should be performed preoperatively rather than postoperatively. Omission of preoperative RT is conditionally recommended in selected patients with lower risk of locoregional recurrence. Addition of chemotherapy before or after chemoradiation or after short-course RT is conditionally recommended. Nonoperative management is conditionally recommended if a clinical complete response is achieved after neoadjuvant treatment in selected patients. Inclusion of the rectum and mesorectal, presacral, internal iliac, and obturator nodes in the clinical treatment volume is recommended. In addition, inclusion of external iliac nodes is conditionally recommended in patients with tumors invading an anterior organ or structure, and inclusion of inguinal and external iliac nodes is conditionally recommended in patients with tumors involving the anal canal.
CONCLUSIONS: Based on currently published data, the American Society for Radiation Oncology task force has proposed evidence-based recommendations regarding the use of RT for rectal cancer. Future studies will look to further personalize treatment recommendations to optimize treatment outcomes and quality of life.
PMID:33097436 | DOI:10.1016/j.prro.2020.08.004
View details for PubMedID 33097436
-
More
-
Validation of a modern second-check dosimetry system using a novel verification phantom Journal of applied clinical medical physics
McDonald DG, Jacqmin DJ, Mart CJ, Koch NC, Peng JL, Ashenafi MS, Fugal MA, Vanek KN
2017 Jan;18(1):170-177. doi: 10.1002/acm2.12025.
-
More
PURPOSE: To evaluate the Mobius second-check dosimetry system by comparing it to ionization-chamber dose measurements collected in the recently released Mobius Verification Phantom™ (MVP). For reference, a comparison of these measurements to dose calculated in the primary treatment planning system (TPS), Varian Eclipse with the AcurosXB dose algorithm, is also provided. Finally, patient dose calculated in Mobius is compared directly to Eclipse to demonstrate typical expected results during clinical use of the Mobius system.
METHODS: Seventeen anonymized intensity-modulated clinical treatment plans were selected for analysis. Dose was recalculated on the MVP in both Eclipse and Mobius. These calculated doses were compared to doses measured using an A1SL ionization-chamber in the MVP. Dose was measured and analyzed at two different chamber positions for each treatment plan. Mobius calculated dose was then compared directly to Eclipse using the following metrics; target mean dose, target D95%, global 3D gamma pass rate, and target gamma pass rate. Finally, these same metrics were used to analyze the first 36 intensity modulated cases, following clinical implementation of the Mobius system.
RESULTS: The average difference between Mobius and measurement was 0.3 ± 1.3%. Differences ranged from -3.3 to + 2.2%. The average difference between Eclipse and measurement was -1.2 ± 0.7%. Eclipse vs. measurement differences ranged from -3.0 to -0.1%. For the 17 anonymized pre-clinical cases, the average target mean dose difference between Mobius and Eclipse was 1.0 ± 1.1%. Average target D95% difference was -0.9 ± 2.0%. Average global gamma pass rate, using a criteria of 3%, 2 mm, was 94.4 ± 3.3%, and average gamma pass rate for the target volume only was 80.2 ± 12.3%. Results of the first 36 intensity-modulated cases, post-clinical implementation of Mobius, were similar to those seen for the 17 pre-clinical test cases.
CONCLUSION: Mobius correctly calculated dose for each tested intensity modulated treatment plan, agreeing with measurement to within 3.5% for all cases analyzed. The dose calculation accuracy and independence of the Mobius system is sufficient to provide a rigorous second-check of a modern TPS.
PMID:28291938 | PMC:PMC5689885 | DOI:10.1002/acm2.12025
View details for PubMedID 28291938
-
More
-
Implementation of the validation testing in MPPG 5.a "Commissioning and QA of treatment planning dose calculations-megavoltage photon and electron beams" Journal of applied clinical medical physics
Jacqmin DJ, Bredfeldt JS, Frigo SP, Smilowitz JB
2017 Jan;18(1):115-127. doi: 10.1002/acm2.12015. Epub 2016 Dec 5.
-
More
The AAPM Medical Physics Practice Guideline (MPPG) 5.a provides concise guidance on the commissioning and QA of beam modeling and dose calculation in radiotherapy treatment planning systems. This work discusses the implementation of the validation testing recommended in MPPG 5.a at two institutions. The two institutions worked collaboratively to create a common set of treatment fields and analysis tools to deliver and analyze the validation tests. This included the development of a novel, open-source software tool to compare scanning water tank measurements to 3D DICOM-RT Dose distributions. Dose calculation algorithms in both Pinnacle and Eclipse were tested with MPPG 5.a to validate the modeling of Varian TrueBeam linear accelerators. The validation process resulted in more than 200 water tank scans and more than 50 point measurements per institution, each of which was compared to a dose calculation from the institution's treatment planning system (TPS). Overall, the validation testing recommended in MPPG 5.a took approximately 79 person-hours for a machine with four photon and five electron energies for a single TPS. Of the 79 person-hours, 26 person-hours required time on the machine, and the remainder involved preparation and analysis. The basic photon, electron, and heterogeneity correction tests were evaluated with the tolerances in MPPG 5.a, and the tolerances were met for all tests. The MPPG 5.a evaluation criteria were used to assess the small field and IMRT/VMAT validation tests. Both institutions found the use of MPPG 5.a to be a valuable resource during the commissioning process. The validation testing in MPPG 5.a showed the strengths and limitations of the TPS models. In addition, the data collected during the validation testing is useful for routine QA of the TPS, validation of software upgrades, and commissioning of new algorithms.
PMID:28291929 | PMC:PMC5689890 | DOI:10.1002/acm2.12015
View details for PubMedID 28291929
-
More
-
RIP1 and RIP3 complex regulates radiation-induced programmed necrosis in glioblastoma Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine
Das A, McDonald DG, Dixon-Mah YN, Jacqmin DJ, Samant VN, Vandergrift WA, Lindhorst SM, Cachia D, Varma AK, Vanek KN, Banik NL, Jenrette JM, Raizer JJ, Giglio P, Patel SJ
2016 Jun;37(6):7525-34. doi: 10.1007/s13277-015-4621-6. Epub 2015 Dec 18.
-
More
Radiation-induced necrosis (RN) is a relatively common side effect of radiation therapy for glioblastoma. However, the molecular mechanisms involved and the ways RN mechanisms differ from regulated cell death (apoptosis) are not well understood. Here, we compare the molecular mechanism of cell death (apoptosis or necrosis) of C6 glioma cells in both in vitro and in vivo (C6 othotopically allograft) models in response to low and high doses of X-ray radiation. Lower radiation doses were used to induce apoptosis, while high-dose levels were chosen to induce radiation necrosis. Our results demonstrate that active caspase-8 in this complex I induces apoptosis in response to low-dose radiation and inhibits necrosis by cleaving RIP1 and RI. When activation of caspase-8 was reduced at high doses of X-ray radiation, the RIP1/RIP3 necrosome complex II is formed. These complexes induce necrosis through the caspase-3-independent pathway mediated by calpain, cathepsin B/D, and apoptosis-inducing factor (AIF). AIF has a dual role in apoptosis and necrosis. At high doses, AIF promotes chromatinolysis and necrosis by interacting with histone H2AX. In addition, NF-κB, STAT-3, and HIF-1 play a crucial role in radiation-induced inflammatory responses embedded in a complex inflammatory network. Analysis of inflammatory markers in matched plasma and cerebrospinal fluid (CSF) isolated from in vivo specimens demonstrated the upregulation of chemokines and cytokines during the necrosis phase. Using RIP1/RIP3 kinase specific inhibitors (Nec-1, GSK'872), we also establish that the RIP1-RIP3 complex regulates programmed necrosis after either high-dose radiation or TNF-α-induced necrosis requires RIP1 and RIP3 kinases. Overall, our data shed new light on the relationship between RIP1/RIP3-mediated programmed necrosis and AIF-mediated caspase-independent programmed necrosis in glioblastoma.
PMID:26684801 | DOI:10.1007/s13277-015-4621-6
View details for PubMedID 26684801
-
More
-
Complement-dependent modulation of antitumor immunity following radiation therapy Cell reports
Elvington M, Scheiber M, Yang X, Lyons K, Jacqmin D, Wadsworth C, Marshall D, Vanek K, Tomlinson S
2014 Aug 7;8(3):818-30. doi: 10.1016/j.celrep.2014.06.051. Epub 2014 Jul 24.
-
More
Complement is traditionally thought of as a proinflammatory effector mechanism of antitumor immunity. However, complement is also important for effective clearance of apoptotic cells, which can be an anti-inflammatory and tolerogenic process. We show that localized fractionated radiation therapy (RT) of subcutaneous murine lymphoma results in tumor cell apoptosis and local complement activation. Cotreatment of mice with tumor-targeted complement inhibition markedly improved therapeutic outcome of RT, an effect linked to early increases in apoptotic cell numbers and increased inflammation. Improved outcome was dependent on an early neutrophil influx and was characterized by increased numbers of mature dendritic cells and the subsequent modulation of T cell immunity. Appropriate complement inhibition may be a promising strategy to enhance a mainstay of treatment for cancer.
PMID:25066124 | PMC:PMC4137409 | DOI:10.1016/j.celrep.2014.06.051
View details for PubMedID 25066124
-
More
Contact Information
Dustin Jacqmin, PhD
600 Highland Avenue,K4/B69
Madison, WI 53792