I am an associate professor in the Department of Human Oncology. My clinical focus is on brachytherapy and imaging for radiation therapy, specifically CT simulation. CT imaging has made great advances, such as dual-energy CT, and I am passionate about understanding the ways in which these technologies can benefit our patients.
In addition to my clinical and research roles, I also enjoy the many teaching opportunities available at the University of Wisconsin. I teach a graduate-level brachytherapy course for the Department of Medical Physics. Within the Department of Human Oncology, I serve as a mentor for the medical physics residency program. I focus on training residents in the physics of different dosimeters and imaging techniques.
Education
PhD, University of Wisconsin–Madison, Medical Physics (2013)
MS, University of Wisconsin–Madison, Medical Physics (2009)
BA/BS, University of Idaho and University of the Basque Country, Physics and Spanish (2007)
Academic Appointments
Associate Professor (CHS), Human Oncology (2023)
Assistant Professor (CHS), Human Oncology (2015)
Assistant Researcher, Human Oncology (2013–2015)
Research Assistant, Medical Physics (2007–2013)
Selected Honors and Awards
Editor’s pick for the Journal of Medical Physics (2016)
University of Wisconsin Ride Scholar (2016)
First Place, Young Investigator Competition at NCC of the AAPM Meeting (2013)
First Place, Young Investigator Competition at NCC of the AAPM Meeting (2009)
Student Travel Grant Award Winner, Annual Meeting of the CIRMS (2009)
Physics Student of the Year, University of Idaho (2007)
Leonard Halland Physics Scholarship (2007)
College of Science Dean’s List (2003–2007)
University of Idaho Honors Scholarship (2003–2007)
Idaho Academic Scholarship (2003–2007)
Boards, Advisory Committees and Professional Organizations
Accredited Dosimetry Calibration Laboratory Advisory Board Committee Member (2017–pres.)
CT Simulation Improvement Committee Chair (2017-pres.)
Medical Physics Residency Advisory Committee Member (2017–pres.)
Doctoral Candidate Research Advisor (2017–pres.)
Doctoral Candidate Committee Member (2016–pres.)
Radiation Oncology Residency Program Selection Committee Member (2016–pres.)
Medical Physics Residency Program Selection Committee Member (2016–pres.)
Medical Physics Residency Program Curriculum Committee Member (2016–pres.)
UW–La Crosse Medical Dosimetry Program Selection Committee (2014–2015)
UWMRRC Research Oversight Committee (2010–2013)
American Association of Physicists in Medicine (2008–pres.)
Council of Ionization Radiation Measurements (2008–2013)
Research Focus
Brachytherapy, Clinical Operations, Imaging, Motion Management, Radiation Measurements
Dr. Jessica Miller focuses on brachytherapy and imaging for radiation therapy, specifically CT simulation. She serves as a mentor for the medical physics residency program, training residents in the physics of different dosimeters and imaging techniques. She also teaches a graduate-level brachytherapy course for the Department of Medical Physics.
Research is a critical component to implementing new technologies that allow us to improve care for our patients in the fight against cancer.
Radiation therapy relies on an accurate understanding of tumor location within a patient as well as an accurate model of the radiation beam to be delivered. My research focuses on both of these aspects of radiation therapy delivery. Through optimized CT imaging we can better define the boundaries of a tumor and target the cancer more effectively. I also work with radiation detectors to characterize their response to radiation for a more accurate measurement of dose.
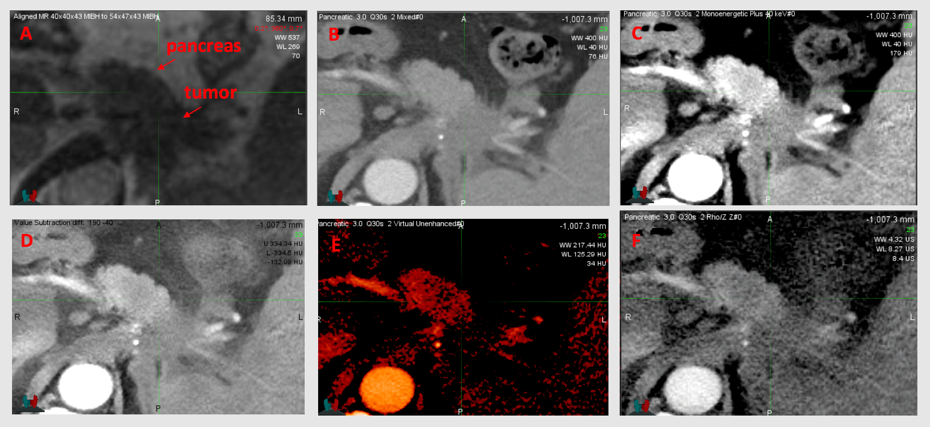
Improving tumor delineation in the pancreas and liver through dual-energy CT
Tumor delineation in the pancreas and liver can be a challenge using conventional CT images. Dual-Energy CT provides many opportunities to better delineate tumor from healthy tissue and therefore has great potential to aid in radiation therapy. The Department of Human Oncology has installed a novel single-source dual-energy CT system, called TwinBeam, with potential for liver and pancreas imaging. We are currently quantifying the advantages gained through TwinBeam dual-energy CT.
Polarity and ion recombination effects in Microionization chambers
An increase in the delivery of small and nonstandard radiation fields has led to the development of small-volume ionization chambers, commonly categorized as microchambers. Small-volume dosimeters can provide high spatial resolution in areas of steep dose gradients. The University of Wisconsin Accredited Dosimetry Calibration Laboratory has experienced an increase in requests for the calibration of microchambers. This indicates that these chambers are being used for reference dosimetry measurements in a wide range of therapy applications. Unfortunately, microchambers exhibit anomalous polarity and ion recombination effects that are not demonstrated by larger-volume, reference-class ionization chambers. We are working to better understand and characterize these chambers.
-
Intratumoral radiation dose heterogeneity augments antitumor immunity in mice and primes responses to checkpoint blockade Science translational medicine
Jagodinsky JC, Vera JM, Jin WJ, Shea AG, Clark PA, Sriramaneni RN, Havighurst TC, Chakravarthy I, Allawi RH, Kim K, Harari PM, Sondel PM, Newton MA, Crittenden MR, Gough MJ, Miller JR, Ong IM, Morris ZS
2024 Sep 18;16(765):eadk0642. doi: 10.1126/scitranslmed.adk0642. Epub 2024 Sep 18.
-
More
Radiation therapy (RT) activates multiple immunologic effects in the tumor microenvironment (TME), with diverse dose-response relationships observed. We hypothesized that, in contrast with homogeneous RT, a heterogeneous RT dose would simultaneously optimize activation of multiple immunogenic effects in a single TME, resulting in a more effective antitumor immune response. Using high-dose-rate brachytherapy, we treated mice bearing syngeneic tumors with a single fraction of heterogeneous RT at a dose ranging from 2 to 30 gray. When combined with dual immune checkpoint inhibition in murine models, heterogeneous RT generated more potent antitumor responses in distant, nonirradiated tumors compared with any homogeneous dose. The antitumor effect after heterogeneous RT required CD4 and CD8 T cells and low-dose RT to a portion of the tumor. At the 3-day post-RT time point, dose heterogeneity imprinted the targeted TME with spatial differences in immune-related gene expression, antigen presentation, and susceptibility of tumor cells to immune-mediated destruction. At a later 10-day post-RT time point, high-, moderate-, or low-RT-dose regions demonstrated distinct infiltrating immune cell populations. This was associated with an increase in the expression of effector-associated cytokines in circulating CD8 T cells. Consistent with enhanced adaptive immune priming, heterogeneous RT promoted clonal expansion of effector CD8 T cells. These findings illuminate the breadth of dose-dependent effects of RT on the TME and the capacity of heterogeneous RT to promote antitumor immunity when combined with immune checkpoint inhibitors.
PMID:39292804 | PMC:PMC11522033 | DOI:10.1126/scitranslmed.adk0642
View details for PubMedID 39292804
-
More
-
Dosimetric evaluation of adaptive planning for five-fraction gynecologic template-based interstitial brachytherapy Brachytherapy
Blum S, Miller JR, Bradley KA, Anderson B, Menon H, Eckelmann B, Wallace C, Besemer A, Lawless M, Slagowski JM
2024 Nov-Dec;23(6):668-675. doi: 10.1016/j.brachy.2024.07.007. Epub 2024 Aug 31.
-
More
PURPOSE: The purpose of this work was to evaluate whether inter-fraction imaging and replanning enhance treatment delivery adherence to clinical planning objectives in the context of a 5-fraction template-based interstitial brachytherapy (TISB) approach for gynecologic cancer treatment.
METHODS AND MATERIALS: This retrospective study analyzed nineteen patients who underwent 5 fractions of interstitial brachytherapy over 3 days using the Syed-Neblett template. A verification CT scan was acquired for applicator assessment and reviewed by a radiation oncologist and medical physicist before each fraction. Eleven patients required replanning at least once during the treatment course. Replanning on the verification CT scan consisted of generating new target and organ-at-risk contours, digitizing catheter positions, and optimizing source dwell times to meet planning objectives. Dwell times and positions from the initial treatment plan were evaluated on the new contours to assess the dose that would have been delivered without replanning (nonadapted). Significance of nonadapted versus adapted dose differences were evaluated using a 2-sided Wilcoxon sum rank test.
RESULTS: The average (min, max) change in dose (Gy) between the clinically delivered plans and the nonadapted plans were HR-CTV D90%: -6.5 (-0.6, -15.1), HR-CTV D98%: -6.5 (-0.4, -12.6), Bladder D2cc: -0.5 (0.0, -2.8), Bowel D2cc: -0.8 (0.0, -3.2), Rectum D2cc: -1.1 (0.0, -11.5), Sigmoid D2cc: -1.4 (-0.1, -5.4). Dosimetric changes in HR-CTV coverage were significantly improved with replanning while organ-at-risk differences were nonsignificant (p > 0.05). Fraction 3 was the most common fraction indicated for replanning.
CONCLUSIONS: Replanning template-based interstitial brachytherapy can improve target coverage and adherence to planning goals.
PMID:39217003 | DOI:10.1016/j.brachy.2024.07.007
View details for PubMedID 39217003
-
More
-
Evaluating on-board kVCT- and MVCT-based dose calculation accuracy using a thorax phantom for helical tomotherapy treatments Biomedical physics & engineering express
Tegtmeier RC, Ferris WS, Chen R, Miller JR, Bayouth JE, Culberson WS
2023 Feb 14;9(2). doi: 10.1088/2057-1976/acb93f.
-
More
Purpose.To evaluate the impact of CT number calibration and imaging parameter selection on dose calculation accuracy relative to the CT planning process in thoracic treatments for on-board helical CT imaging systems used in helical tomotherapy.Methods and Materials.Direct CT number calibrations were performed with appropriate protocols for each imaging system using an electron density phantom. Large volume and SBRT treatment plans were simulated and optimized for planning CT scans of an anthropomorphic thorax phantom and transferred to registered kVCT and MVCT scans of the phantom as appropriate. Relevant DVH metrics and dose-difference maps were used to evaluate and compare dose calculation accuracy relative to the planning CT based on a variation in imaging parameters applied for the on-board systems.Results.For helical kVCT scans of the thorax phantom, median differences in DVH parameters for the large volume treatment plan were less than ±1% with dose to the target volume either over- or underestimated depending on the imaging parameters utilized for CT number calibration and thorax phantom acquisition. For the lung SBRT plan calculated on helical kVCT scans, median dose differences were up to -2.7% with a more noticeable dependence on parameter selection. For MVCT scans, median dose differences for the large volume plan were within +2% with dose to the target overestimated regardless of the imaging protocol.Conclusion.Accurate dose calculations (median errors of <±1%) using a thorax phantom simulating realistic patient geometry and scatter conditions can be achieved with images acquired with a helical kVCT system on a helical tomotherapy unit. This accuracy is considerably improved relative to that achieved with the MV-based approach. In a clinical setting, careful consideration should be made when selecting appropriate kVCT imaging parameters for this process as dose calculation accuracy was observed to vary with both parameter selection and treatment type.
PMID:36745904 | DOI:10.1088/2057-1976/acb93f
View details for PubMedID 36745904
-
More
-
Targeting the GTV in medically inoperable endometrial cancer using brachytherapy Brachytherapy
Merfeld EC, Kuczmarska-Haas A, Burr AR, Witt JS, Francis DM, Ntambi J, Desai VK, Huang JY, Miller JR, Lawless MJ, Wallace CR, Anderson BM, Bradley KA
2022 Nov-Dec;21(6):792-798. doi: 10.1016/j.brachy.2022.07.006. Epub 2022 Aug 24.
-
More
PURPOSE: We aimed to determine the relationship between gross tumor volume (GTV) dose and tumor control in women with medically inoperable endometrial cancer, and to demonstrate the feasibility of targeting a GTV-focused volume using imaged-guided brachytherapy.
METHODS AND MATERIALS: An endometrial cancer database was used to identify patients. Treatment plans were reviewed to determine doses to GTV, clinical target volume (CTV), and OARs. Uterine recurrence-free survival was evaluated as a function of CTV and GTV doses. Brachytherapy was replanned with a goal of GTV D98 EQD2 ≥ 80 Gy, without regard for coverage of the uninvolved uterus and while respecting OAR dose constraints.
RESULTS: Fifty-four patients were identified. In the delivered plans, GTV D90 EQD2 ≥ 80 Gy was achieved in 36 (81.8%) patients. Uterine recurrence-free survival was 100% in patients with GTV D90 EQD2 ≥ 80 Gy and 66.7% in patients with EQD2 < 80 Gy (p = 0.001). On GTV-only replans, GTV D98 EQD2 ≥ 80 Gy was achieved in 39 (88.6%) patients. Mean D2cc was lower for bladder (47.1 Gy vs. 73.0 Gy, p < 0.001), and sigmoid (47.0 Gy vs. 58.0 Gy, p = 0.007) on GTV-only replans compared to delivered plans. Bladder D2cc was ≥ 80 Gy in 11 (25.0%) delivered plans and four (9.1%) GTV-only replans (p = 0.043). Sigmoid D2cc was ≥ 65 Gy in 20 (45.4%) delivered plans and 10 (22.7%) GTV-only replans (p = 0.021).
CONCLUSIONS: OAR dose constraints should be prioritized over CTV coverage if GTV coverage is sufficient. Prospective evaluation of image-guided brachytherapy to a reduced, GTV-focused volume is warranted.
PMID:36030167 | DOI:10.1016/j.brachy.2022.07.006
View details for PubMedID 36030167
-
More
-
Commissioning an Exradin W2 plastic scintillation detector for clinical use in small radiation fields Journal of applied clinical medical physics
Jacqmin DJ, Miller JR, Barraclough BA, Labby ZE
2022 Aug;23(8):e13728. doi: 10.1002/acm2.13728. Epub 2022 Jul 21.
-
More
PURPOSE: The purpose of this work is to evaluate the Standard Imaging Exradin W2 plastic scintillation detector (W2) for use in the types of fields used for stereotactic radiosurgery.
METHODS: Prior to testing the W2 in small fields, the W2 was evaluated in standard large field conditions to ensure good detector performance. These tests included energy dependence, short-term repeatability, dose-response linearity, angular dependence, temperature dependence, and dose rate dependence. Next, scan settings and calibration of the W2 were optimized to ensure high quality data acquisition. Profiles of small fields shaped by cones and multi-leaf collimator (MLCs) were measured using the W2 and IBA RAZOR diode in a scanning water tank. Output factors for cones (4-17.5 mm) and MLC fields (1, 2, 3 cm) were acquired with both detectors. Finally, the dose at isocenter for seven radiosurgery plans was measured with the W2 detector.
RESULTS: W2 exhibited acceptable warm-up behavior, short-term reproducibility, axial angular dependence, dose-rate linearity, and dose linearity. The detector exhibits a dependence upon energy, polar angle, and temperature. Scanning measurements taken with the W2 and RAZOR were in good agreement, with full-width half-maximum and penumbra widths agreeing to within 0.1 mm. The output factors measured by the W2 and RAZOR exhibited a maximum difference of 1.8%. For the seven point-dose measurements of radiosurgery plans, the W2 agreed well with our treatment planning system with a maximum deviation of 2.2%. The Čerenkov light ratio calibration method did not significantly impact the measurement of relative profiles, output factors, or point dose measurements.
CONCLUSION: The W2 demonstrated dosimetric characteristics that are suitable for radiosurgery field measurements. The detector agreed well with the RAZOR diode for output factors and scanned profiles and showed good agreement with the treatment planning system in measurements of clinical treatment plans.
PMID:35861648 | PMC:PMC9359019 | DOI:10.1002/acm2.13728
View details for PubMedID 35861648
-
More
-
Characterization of imaging performance of a novel helical kVCT for use in image-guided and adaptive radiotherapy Journal of applied clinical medical physics
Tegtmeier RC, Ferris WS, Bayouth JE, Miller JR, Culberson WS
2022 Jun;23(6):e13648. doi: 10.1002/acm2.13648. Epub 2022 May 15.
-
More
ClearRT helical kVCT imaging for the Radixact helical tomotherapy system recently received FDA approval and is available for clinical use. The system is intended to enhance image fidelity in radiation therapy treatment planning and delivery compared to the prior MV-based onboard imaging approach. The purpose of this work was to characterize the imaging performance of this system and compare this performance with that of clinical systems used in image-guided and/or adaptive radiotherapy (ART) or computed tomography (CT) simulation, including Radixact MVCT, TomoTherapy MVCT, Varian TrueBeam kV OBI CBCT, and the Siemens SOMATOM Definition Edge kVCT. A CT image quality phantom was scanned across clinically relevant acquisition modes for each system to evaluate image quality metrics, including noise, uniformity, contrast, spatial resolution, and CT number linearity. Similar noise levels were observed for ClearRT and Siemens Edge, whereas noise for the other systems was ∼1.5-5 times higher. Uniformity was best for Siemens Edge, whereas most scans for ClearRT exhibited a slight "cupping" or "capping" artifact. The ClearRT and Siemens Edge performed best for contrast metrics, which included low-contrast visibility and contrast-to-noise ratio evaluations. Spatial resolution was best for TrueBeam and Siemens Edge, whereas the three kVCT systems exhibited similar CT number linearity. Overall, these results provide an initial indication that ClearRT image quality is adequate for image guidance in radiotherapy and sufficient for delineating anatomic structures, thus enabling its use for ART. ClearRT also showed significant improvement over MVCT, which was previously the only onboard imaging modality available on Radixact. Although the acquisition of these scans does come at the cost of additional patient dose, reported CTDI values indicate a similar or generally reduced machine output for ClearRT compared to the other systems while maintaining comparable or improved image quality overall.
PMID:35570390 | PMC:PMC9194993 | DOI:10.1002/acm2.13648
View details for PubMedID 35570390
-
More
-
Stereotactic Radiation Therapy for an Arteriovenous Malformation of the Oral Tongue: A Teaching Case Advances in radiation oncology
Merfeld EC, Labby ZE, Miller JR, Burr AR, Wong F, Diamond C, Wieland AR, Aagaard-Kienitz B, Howard SP
2021 Dec 16;7(3):100870. doi: 10.1016/j.adro.2021.100870. eCollection 2022 May-Jun.
-
A multipurpose brachytherapy catheter to enable intratumoral injection Brachytherapy
Jagodinsky JC, Medeiros G, Raj HH, Razuan A, Locsin A, Dempsey TG, Tang B, Chakravarty I, Clark PA, Sriramaneni RN, Jin WJ, Lan K, Das RK, Miller JR, Suarez-Gonzalez D, Morris ZS
2021 Jul-Aug;20(4):900-910. doi: 10.1016/j.brachy.2020.10.012. Epub 2021 Mar 27.
-
More
PURPOSE: To create and test a multipurpose brachytherapy catheter prototype enabling intratumoral injection and brachytherapy after a single catheter insertion.
METHODS AND MATERIALS: The design of the prototype consists of an outer tube and an inner syringe tube that can be filled with injectable agent. The outer sheath and inner syringe tube were constructed using polytetrafluoroethylene tubing, and the other components were 3D printed using dental resin and polylactic acid material. To demonstrate functionality, we injected in vitro phantoms with dyed saline. For proof of concept, we demonstrated the potential for the prototype to deliver cell therapy, enhance tumor delineation, deliver tattoo ink for pathology marking, avoid toxicity through local delivery of chemotherapy, and facilitate combination brachytherapy and immunotherapy.
RESULTS: The prototype enables accurate injection in vitro and in vivo without altering dosimetry. To illustrate the potential for delivery of cell therapies, we injected luciferase-expressing splenocytes and confirmed their delivery with bioluminescence imaging. To demonstrate feasibility of radiographically visualizing injected material, we delivered iohexol contrast intratumorally and confirmed tumor retention using Faxitron x-ray imaging. In addition, we show the potential of intratumoral administration to reduce toxicity associated with cyclophosphamide compared with systemic administration. To demonstrate feasibility, we treated tumor-bearing mice with brachytherapy (192Ir source, 2 Gy to 5 mm) in combination with intratumoral injection of 375,000 U of interleukin 2 and observed no increased toxicity.
CONCLUSIONS: These results demonstrate that a prototype multipurpose brachytherapy catheter enables accurate intratumoral injection and support the feasibility of combining intratumoral injection with brachytherapy.
PMID:33785280 | PMC:PMC8323107 | DOI:10.1016/j.brachy.2020.10.012
View details for PubMedID 33785280
-
More
-
Evaluation of a commercial deformable image registration algorithm for dual-energy CT processing Journal of applied clinical medical physics
Huang JY, Lawless MJ, Matrosic CK, Maso DD, Miller JR
2020 Sep;21(9):227-234. doi: 10.1002/acm2.12987. Epub 2020 Jul 25.
-
More
PURPOSE: Several dual-energy computed tomography (DECT) techniques require a deformable image registration to correct for motion between the acquisition of low and high energy data. However, current DECT software does not provide tools to assess registration accuracy or allow the user to export deformed images, presenting a unique challenge for image registration quality assurance (QA). This work presents a methodology to evaluate the accuracy of DECT deformable registration and to quantify the impact of registration errors on end-product images.
METHODS: The deformable algorithm implemented in Siemen Healthineers's Syngo was evaluated using a deformable abdomen phantom and a rigid phantom to mimic sliding motion in the thorax. Both phantoms were imaged using sequential 80 and 140 kVp scans with motion applied between the two scans. Since Syngo does not allow the export of the deformed images, this study focused on quantifying the accuracy of various end-product, dual-energy images resulting from processing of deformed images.
RESULTS: The Syngo algorithm performed well for the abdomen phantom with a mean registration error of 0.4 mm for landmark analysis, Dice similarity coefficients (DSCs) > 0.90 for five organs contoured, and mean iodine concentrations within 0.2 mg/mL of values measured on static images. For rigid sliding motion, the algorithm performed poorer and resulted in noticeable registration errors toward the superior and inferior scan extents and DSCs as low as 0.41 for iodine rods imaged in the phantom. Additionally, local iodine concentration errors in areas of misregistration exceeded 3 mg/mL.
CONCLUSIONS: This work represents the first methodology for DECT image registration QA using commercial software. Our data support the clinical use of the Syngo algorithm for abdominal sites with limited motion (i.e., pancreas and liver). However, dual-energy images generated with this algorithm should be used with caution for quantitative measurements in areas with sliding motion.
PMID:32710502 | PMC:PMC7497912 | DOI:10.1002/acm2.12987
View details for PubMedID 32710502
-
More
-
Investigating split-filter dual-energy CT for improving liver tumor visibility for radiation therapy Journal of applied clinical medical physics
DiMaso LD, Miller JR, Lawless MJ, Bassetti MF, DeWerd LA, Huang J
2020 Aug;21(8):249-255. doi: 10.1002/acm2.12904. Epub 2020 May 15.
-
More
PURPOSE: Accurate liver tumor delineation is crucial for radiation therapy, but liver tumor volumes are difficult to visualize with conventional single-energy CT. This work investigates the use of split-filter dual-energy CT (DECT) for liver tumor visibility by quantifying contrast and contrast-to-noise ratio (CNR).
METHODS: Split-filter DECT contrast-enhanced scans of 20 liver tumors including cholangiocarcinomas, hepatocellular carcinomas, and liver metastases were acquired. Analysis was performed on the arterial and venous phases of mixed 120 kVp-equivalent images and VMIs at 57 keV and 40 keV gross target volume (GTV) contrast and CNR were calculated.
RESULTS: For the arterial phase, liver GTV contrast was 12.1 ± 10.0 HU and 43.1 ± 32.3 HU (P < 0.001) for the mixed images and 40 keV VMIs. Image noise increased on average by 116% for the 40 keV VMIs compared to the mixed images. The average CNR did not change significantly (1.6 ± 1.5, 1.7 ± 1.4, 2.4 ± 1.7 for the mixed, 57 keV and 40 keV VMIs (P > 0.141)). For individual cases, however, CNR increases of up to 607% were measured for the 40 keV VMIs compared to the mixed image. Venous phase 40 keV VMIs demonstrated an average increase of 35.4 HU in GTV contrast and 121% increase in image noise. Average CNR values were also not statistically different, but for individual cases CNR increases of up to 554% were measured for the 40 keV VMIs compared to the mixed image.
CONCLUSIONS: Liver tumor contrast was significantly improved using split-filter DECT 40 keV VMIs compared to mixed images. On average, there was no statistical difference in CNR between the mixed images and VMIs, but for individual cases, CNR was greatly increased for the 57 keV and 40 keV VMIs. Therefore, although not universally successful for our patient cohort, split-filter DECT VMIs may provide substantial gains in tumor visibility of certain liver cases for radiation therapy treatment planning.
PMID:32410336 | PMC:PMC7484851 | DOI:10.1002/acm2.12904
View details for PubMedID 32410336
-
More
-
Investigating a novel split-filter dual-energy CT technique for improving pancreas tumor visibility for radiation therapy Journal of applied clinical medical physics
Maso DD, Huang J, Bassetti MF, DeWerd LA, Miller JR
2018 Sep;19(5):676-683. doi: 10.1002/acm2.12435. Epub 2018 Aug 17.
-
More
PURPOSE: Tumor delineation using conventional CT images can be a challenge for pancreatic adenocarcinoma where contrast between the tumor and surrounding healthy tissue is low. This work investigates the ability of a split-filter dual-energy CT (DECT) system to improve pancreatic tumor contrast and contrast-to-noise ratio (CNR) for radiation therapy treatment planning.
MATERIALS AND METHODS: Multiphasic scans of 20 pancreatic tumors were acquired using a split-filter DECT technique with iodinated contrast medium, OMNIPAQUETM . Analysis was performed on the pancreatic and portal venous phases for several types of DECT images. Pancreatic gross target volume (GTV) contrast and CNR were calculated and analyzed from mixed 120 kVp-equivalent images and virtual monoenergetic images (VMI) at 57 and 40 keV. The role of iterative reconstruction on DECT images was also investigated. Paired t-tests were used to assess the difference in GTV contrast and CNR among the different images.
RESULTS: The VMIs at 40 keV had a 110% greater image noise compared to the mixed 120 kVp-equivalent images (P < 0.0001). VMIs at 40 keV increased GTV contrast from 15.9 ± 19.9 HU to 93.7 ± 49.6 HU and CNR from 1.37 ± 2.05 to 3.86 ± 2.78 in comparison to the mixed 120 kVp-equivalent images. The iterative reconstruction algorithm investigated decreased noise in the VMIs by about 20% and improved CNR by about 30%.
CONCLUSIONS: Pancreatic tumor contrast and CNR were significantly improved using VMIs reconstructed from the split-filter DECT technique, and the use of iterative reconstruction further improved CNR. This gain in tumor contrast may lead to more accurate tumor delineation for radiation therapy treatment planning.
PMID:30117641 | PMC:PMC6123148 | DOI:10.1002/acm2.12435
View details for PubMedID 30117641
-
More
-
Novel use of ViewRay MRI guidance for high-dose-rate brachytherapy in the treatment of cervical cancer Brachytherapy
Ko HC, Huang JY, Miller JR, Das RK, Wallace CR, Costa AD, Francis DM, Straub MR, Anderson BM, Bradley KA
2018 Jul-Aug;17(4):680-688. doi: 10.1016/j.brachy.2018.04.005. Epub 2018 Jun 7.
-
More
PURPOSE: To characterize image quality and feasibility of using ViewRay MRI (VR)-guided brachytherapy planning for cervical cancer.
METHODS AND MATERIALS: Cervical cancer patients receiving intracavitary brachytherapy with tandem and ovoids, planned using 0.35T VR MRI at our institution, were included in this series. The high-risk clinical target volume (HR-CTV), visible gross tumor volume, bladder, sigmoid, bowel, and rectum contours for each fraction of brachytherapy were evaluated for dosimetric parameters. Typically, five brachytherapy treatments were planned using the T2 sequence on diagnostic MRI for the first and third fractions, and a noncontrast true fast imaging with steady-state precession sequence on VR or CT scan for the remaining fractions. Most patients received 5.5 Gy × 5 fractions using high-dose-rate Ir-192 following 45 Gy of whole-pelvis radiotherapy. The plan was initiated at 5.5 Gy to point A and subsequently optimized and prescribed to the HR-CTV. The goal equivalent dose in 2 Gy fractions for the combined external beam and brachytherapy dose was 85 Gy. Soft-tissue visualization using contrast-to-noise ratios to distinguish normal tissues from tumor at their interface was compared between diagnostic MRI, CT, and VR.
RESULTS: One hundred and forty-two fractions of intracavitary brachytherapy were performed from April 2015 to January 2017 on 29 cervical cancer patients, ranging from stages IB1 to IVA. The median HR-CTV was 27.78 cc, with median D90 HR-CTV of 6.1 Gy. The median time from instrument placement to start of treatment using VR was 65 min (scan time 2 min), compared to 105 min using diagnostic MRI (scan time 11 min) (t-test, p < 0.01). The contrast-to-noise ratio of tumor to cervix in both diagnostic MRI and VR had significantly higher values compared to CT (ANOVA and t-tests, p < 0.01).
CONCLUSIONS: We report the first clinical use of VR-guided brachytherapy. Time to treatment using this approach was shorter compared to diagnostic MRI. VR also provided significant advantage in visualizing the tumor and cervix compared to CT. This presents a feasible and reliable manner to image and plan gynecologic brachytherapy.
PMID:29773331 | DOI:10.1016/j.brachy.2018.04.005
View details for PubMedID 29773331
-
More
-
Reply to: Comment on: polarity effects and apparent ion recombination in microionization chambers [Med. Phys. 43(5) 2141-2152 (2016)] Medical physics
Miller JR, Hooten BD, Micka JA, DeWerd LA
2017 Mar;44(3):1206-1207. doi: 10.1002/mp.12084. Epub 2017 Feb 10.
-
More
We would like to thank Dr. Brivio et al. [Med. Phys.] for their comment on our recent paper. Miller et al. [Med. Phys. 43 (2016) 2141-2152] determined the primary cause of voltage-dependent polarity effects in microchambers to be a potential difference between the guard and collecting electrodes. In their comment, Brivio et al., offer an explanation for the cause of such potential differences. Brivio et al. attribute the potential difference to the disparity in the work functions between guard and collecting electrodes composed of different materials. However, all of the microchambers investigated in Miller et al. contained a guard and collecting electrode which were composed of the same material. Therefore, the explanation offered by Brivio et al. that "the electric potential perturbation arises from the work function difference of the disparate materials electrodes" does not explain the polarity effects exhibited by the microchambers investigated in Miller et al., all of which contain electrodes composed of the same materials.
PMID:28052335 | DOI:10.1002/mp.12084
View details for PubMedID 28052335
-
More
-
Ion recombination and polarity corrections for small-volume ionization chambers in high-dose-rate, flattening-filter-free pulsed photon beams Medical physics
Hyun MA, Miller JR, Micka JA, DeWerd LA
2017 Feb;44(2):618-627. doi: 10.1002/mp.12053. Epub 2017 Feb 2.
-
More
PURPOSE: To investigate ion recombination and polarity effects in scanning and microionization chambers when used with digital electrometers and high-dose-rate linac beams such as flattening-filter-free (FFF) fields, and to compare results against conventional pulsed and continuous photon beams.
METHODS: Saturation curves were obtained for one Farmer-type ionization chamber and eight small-volume chamber models with volumes ranging from 0.01 to 0.13 cm3 using a Varian TrueBeam™ STx with FFF capability. Three beam modes (6 MV, 6 MV FFF, and 10 MV FFF) were investigated, with nominal dose-per-pulse values of 0.0278, 0.0648, and 0.111 cGy/pulse, respectively, at dmax . Saturation curves obtained using the Theratronics T1000 60 Co unit at the UWADCL and a conventional linear accelerator (Varian Clinac iX) were used to establish baseline behavior. Jaffé plots were fitted to obtain Pion , accounting for exponential effects such as charge multiplication. These values were compared with the two-voltage technique recommended in TG-51, and were plotted as a function of dose-per-pulse to assess the ability of small-volume chambers to meet reference-class criteria in FFF beams.
RESULTS: Jaffé- and two-voltage-determined Pion values measured for high-dose-rate beams agreed within 0.1% for the Farmer-type chamber and 1% for scanning and microionization chambers, with the exception of the CC01 which agreed within 2%. With respect to ion recombination and polarity effects, the Farmer-type chamber, scanning chambers and the Exradin A26 microchamber exhibited reference-class behavior in all beams investigated, with the exception of the IBA CC04 scanning chamber, which had an initial recombination correction that varied by 0.2% with polarity. All microchambers investigated, with the exception of the A26, exhibited anomalous polarity and ion recombination behaviors that make them unsuitable for reference dosimetry in conventional and high-dose-rate photon beams.
CONCLUSIONS: The results of this work demonstrate that recombination and polarity behaviors seen in conventional pulsed and continuous photon beams trend accordingly in high-dose-rate FFF linac beams. Several models of small-volume ionization chambers used with a digital electrometer have been shown to meet reference-class requirements with respect to ion recombination and polarity, even in the high-dose-rate environment. For such chambers, a two-voltage technique agreed well with more rigorous methods of determining Pion . However, the results emphasize the need for careful reference detector selection, and indicate that ionization chambers ought to be extensively tested in each beam of interest prior to their use for reference dosimetry.
PMID:28001291 | DOI:10.1002/mp.12053
View details for PubMedID 28001291
-
More
-
Polarity effects and apparent ion recombination in microionization chambers Medical physics
Miller JR, Hooten BD, Micka JA, DeWerd LA
2016 May;43(5):2141. doi: 10.1118/1.4944872.
-
More
PURPOSE: Microchambers demonstrate anomalous voltage-dependent polarity effects. Existing polarity and ion recombination correction factors do not account for these effects. As a result, many commercial microchamber models do not meet the specification of a reference-class ionization chamber as defined by the American Association of Physicists in Medicine. The purpose of this investigation is to determine the cause of these voltage-dependent polarity effects.
METHODS: A series of microchamber prototypes were produced to isolate the source of the voltage-dependent polarity effects. Parameters including ionization-chamber collecting-volume size, stem and cable irradiation, chamber assembly, contaminants, high-Z materials, and individual chamber components were investigated. Measurements were performed with electrodes coated with graphite to isolate electrode conductivity. Chamber response was measured as the potential bias of the guard electrode was altered with respect to the collecting electrode, through the integration of additional power supplies. Ionization chamber models were also simulated using comsol Multiphysics software to investigate the effect of a potential difference between electrodes on electric field lines and collecting volume definition.
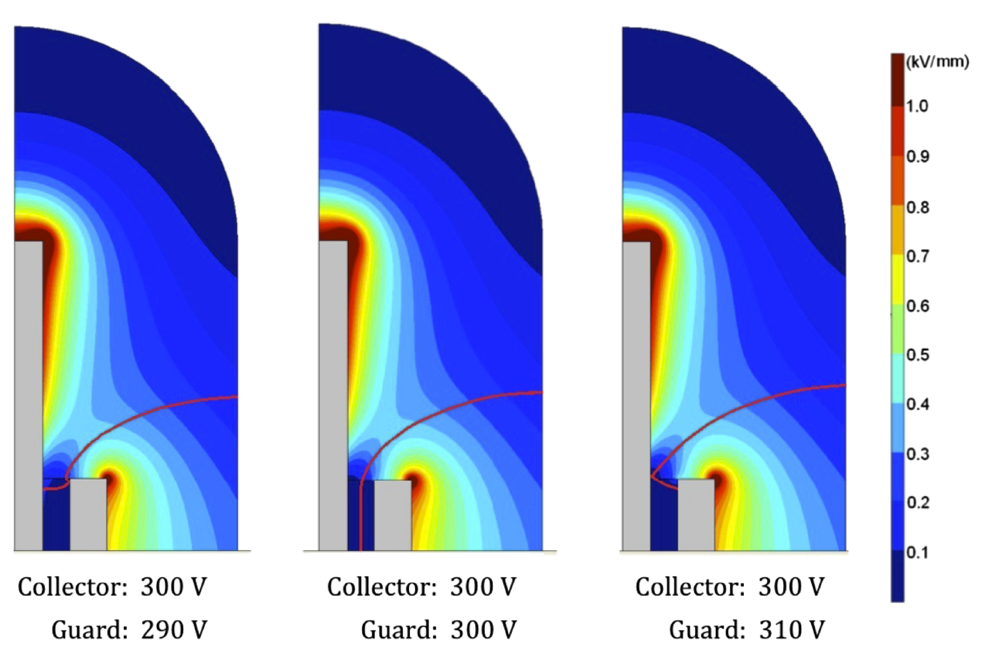
RESULTS: Investigations with microchamber prototypes demonstrated that the significant source of the voltage-dependent polarity effects was a potential difference between the guard and collecting electrodes of the chambers. The voltage-dependent polarity effects for each prototype were primarily isolated to either the guard or collecting electrode. Polarity effects were reduced by coating the isolated electrode with a conductive layer of graphite. Polarity effects were increased by introducing a potential difference between the electrodes. comsol simulations further demonstrated that for a given potential difference between electrodes, the collecting volume of the chamber changed as the applied voltage was altered, producing voltage-dependent polarity effects in the chamber response. Ionization chamber measurements and comsol simulations demonstrated an inverse relationship between the chamber collecting volume size and the severity of voltage-dependent polarity effects on chamber response. The effect of a given potential difference on chamber polarity effects was roughly ten times greater for microchambers as compared to Farmer-type chambers. Stem and cable irradiations, chamber assembly, contaminants, and high-Z materials were not found to be a significant source of the voltage-dependent polarity effects.
CONCLUSIONS: A potential difference between the guard and collecting electrodes was found to be the primary source of the voltage-dependent polarity effects demonstrated by microchambers. For a given potential difference between electrodes, the relative change in the collecting volume is smaller for larger-volume chambers, illustrating why these polarity effects are not seen in larger-volume chambers with similar guard and collecting electrode designs. Thus, for small-volume chambers, it is necessary to reduce the potential difference between the guard and collecting electrodes in order to reduce polarity effects for reference dosimetry measurements.
PMID:27147326 | DOI:10.1118/1.4944872
View details for PubMedID 27147326
-
More
Contact Information
Jessica Miller, PhD
600 Highland Avenue,K4/B86
Madison, WI 53792
