I am an Assistant Professor in the Department of Human Oncology. The study of cancer biology has been my passion since I was an undergraduate when I studied the role of inflammation in lung cancer. I then attended the MSTP (MD/PhD) program at the University of Colorado and worked with HHMI professor Leslie Leinwandduring graduate school. During that time, I established and characterized a model of cardiac muscle atrophy due to cancer. Throughout medical school I loved caring for cancer patients, and combined with my love for cancer research, led me to specialize in radiation oncology.
I moved to St. Louis for my clinical residency at Washington University, where I received excellent training. I was fortunate to receive entry into the B. Leonard Holman Research Pathway during residency, where I performed RNA sequencing of cervical tumors before and during chemoradiation therapy. This research provided novel insight into how these tumors respond to treatment and what factors may be involved in treatment resistance, such as Human Papillomavirus (HPV) oncogene expression and the anti-tumoral immune response. Following residency, I elected to do post-doctoral study here at the University of Wisconsin through the BentsonResearch Fellowship. I worked with Dr. Beth Weaver and studied how chromosomal instability affects radiation sensitivity. This research created the foundation for my own laboratory, where I aim to use a cancer cell’s inherent defects in mitosis as a unique vulnerability to promote cell death.
As a physician-scientist, I treat patients with cancer at the UW Health clinic located in Johnson Creek. My patients are my inspiration for my research, and I hope to discover new ways to sensitize cancer cells to radiation to increase cure.
Education
Bentson Translational Research Fellow, University of Wisconsin, (2018 - 2021)
Resident, Washington University in St. Louis, Radiation Oncology (2018)
Intern, St. Joseph Hospital, Internal Medicine (2014)
MD, University of Colorado, (2013)
PhD, University of Colorado Boulder, Molecular Biology (2011)
BS, University of Texas at Austin, Biochemistry (2002)
Academic Appointments
Assistant Professor, Department of Human Oncology (2022)
Instructor CHS, Department of Human Oncology, (2021-2022)
Fellow, Department of Human Oncology,
Selected Honors and Awards
Forbeck Scholar (2021)
National Institutes of Health (NIH) K08 Career Development Award (2021)
RSNA Fellow Research Grant (2019-2020)
ASCO Young Investigator Award (2019-2020)
RSNA Fellow Research Grant (2019-2020)
RSNA Resident Research Grant (2016-2017)
B. Leonard Holman Research Pathway recipient (2015-2017)
NHLBI Pre-Doctoral T32 Award (2008-2011)
American Association of University Women Scholarship (2010)
American Heart Association Pre-Doctoral Fellowship (2007-2009)
Dow Chemical Centennial Endowed Presidential Scholarship (2002)
Boards, Advisory Committees and Professional Organizations
Associate Senior Editor of Biology, Advances in Radiation Oncology, 2020-pres.
American Society for Radiation Oncology (ASTRO), 2014-pres.
Radiation Research Society (RRS), 2016-pres.
Radiological Society of North America (RSNA), 2016-pres.
American Association for Cancer Research (AACR), 2017-pres.
American Society of Clinical Oncology (ASCO), 2018-pres.
Research Focus
Chromosomal instability, aneuploidy, Head & Neck Cancer and Cervical Cancer
Human Papilloma Virus (HPV) is the main cause of cervical and oropharyngeal cancers. I am studying how chromosomal instability modulates radiation response in HPV-positive and HPV-negative head and neck cancers and how HPV affects the immune response to the tumor.
Chromosomal instability as a mechanism of radiation sensitivity
Chromosomal Instability (CIN) is an ongoing rate of chromosome missegregation events over the course of multiple cell divisions, and is common in human tumors.
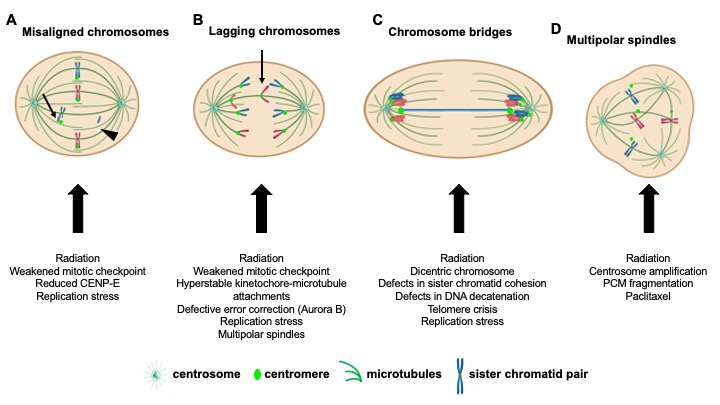
Our laboratory and others have shown that combining two independent insults that each cause tolerable levels of CIN results in high CIN, which leads to cell death and tumor suppression due to loss of essential chromosomes. Ionizing radiation can induce CIN; thus we hypothesize that higher levels of pre-existing CIN in tumors sensitizes cancer cells to radiation therapy.
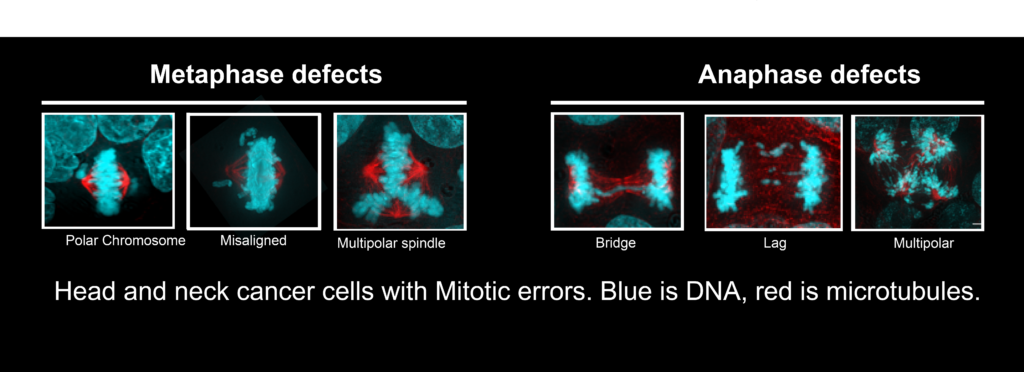
 Just 2 Gy of radiation, the dose normally given to a patient each day during treatment, induces significant mitotic errors, or CIN.
Just 2 Gy of radiation, the dose normally given to a patient each day during treatment, induces significant mitotic errors, or CIN.
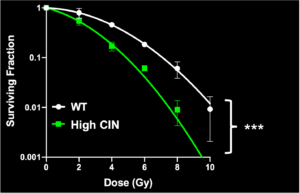 We have engineered cells to have high CIN and found that they indeed were more sensitive to radiation
We have engineered cells to have high CIN and found that they indeed were more sensitive to radiation
We also study how different chemotherapeutics affect CIN and if this could be a novel mechanism of radiosensitization. For example, taxanes have long been thought to increase radiation sensitivity by inducing mitotic arrest. Docetaxel is a taxane used to treat many cancers, including head and neck cancer. We found that when it is used at clinically relevant concentrations, there is no mitotic arrest, and instead, it induces multipolar spindles which leads to cell division in 3 or more directions and cell death. Thus, we discovered a novel mechanism by which docetaxel induces cell death and sensitizes cells to radiation.
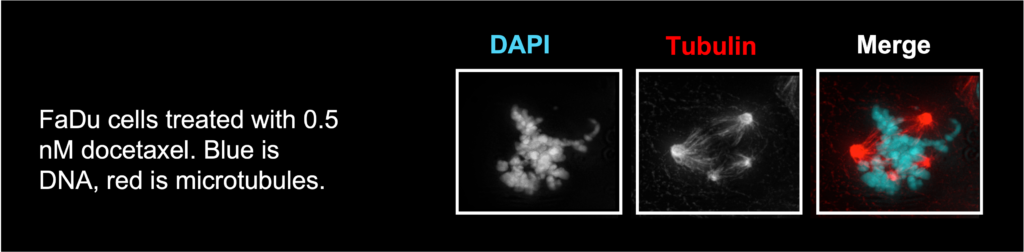
Human Papillomavirus and Chromosomal Instability
Human Papilloma Virus (HPV) is recognized as a significant cause of head and neck cancer and represents a unique clinical entity. Many HPV+ head and neck cancers are more sensitive to radiation then HPV- cancers yet we currently do not have a biomarker to inform us which patients will be cured with a lower dose of radiation, and which patients may need escalated treatment. This is a big issue in radiation oncology because many clinical trials are trying to treat everyone with HPV+ tumors with a lower dose, but we are learning that 10-15% of these patients will have residual or recurrent disease after receiving the lower dose, and hence a poor prognosis. This is a clear example of “one size does not fit all” and it is imperative that we think about the biology of each patient’s tumor to determine the most effective treatment.
Another main interest of my lab is therefore to study how different HPV genotypes (i.e. HPV16 v. HPV18) and different HPV viral genes (i.e. E2, E5, E6, E7) affect CIN. We recently reported that HPV+ head and neck cancers had significantly more misaligned chromosomes than HPV- cancers (A). We found that the HPV oncoprotein E6 causes polar chromosomes (Panels G,H) by causing the specific degradation of the mitotic kinesin CENP-E (panel I). Further work will determine if this has implications for treatment response.
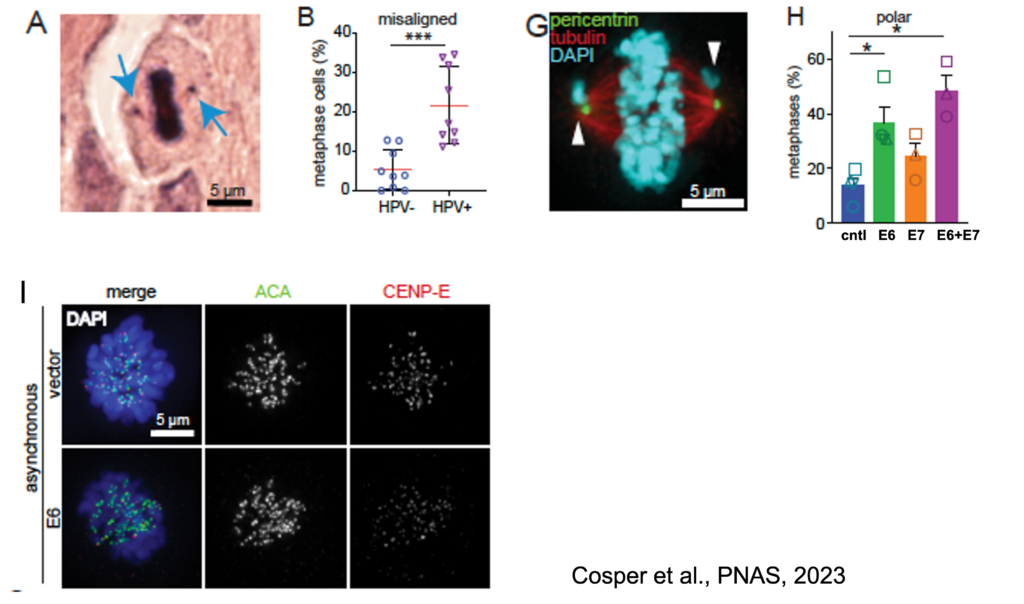
Determine how HPV and CIN affect innate immunity in the context of radiotherapy
Many cell types sense exogenous DNA through the cytosolic DNA receptor GMP-AMP synthase (cGAS), which produces a second messenger cGAMP which activates STING, and ultimately results in a type I interferon immune response. Cells utilize this pathway to protect the host from viral and bacterial infection as well as from genomic instability, which can also cause cytosolic DNA. Many cancer cells have lost the cGAS/STING pathway to avoid immune detection and aid in their survival. I am interested in determining if cancers caused by Human Papilloma Virus (HPV) have differential activation of the cGAS/STING pathway at baseline and in response to radiation compared to HPV negative cancers, and whether this affects the immune response to the tumor. Immunotherapy has revolutionized cancer treatment, but it has not shown robust activity in head and neck or cervical cancer to date. More research is sorely needed to determine the most effective combinations of drugs and radiation. We will be studying how the unique biology of each tumor, such as HPV gene expression and CIN, affects anti-tumoral immunity.
Current Lab Members
Kathryn Jones, M.S. – Lab manager, Research Specialist
Yeseo Choi, Graduate Student, Cancer Biology
Sophie Bice, BS – Medical student (summer 2022, and 2024-2025)
Elizabeth Boulanger – Undergraduate student
Emily McCunn – Undergraduate student
Pate Lantz – Undergraduate student
Ava Bryan – Undergraduate student
Hunter Blalock, BS – Medical student (summer 2023)
Christy Ojeda, BS – Medical student (summer 2023)
Radchanon (Dew) Leelasukseree – Undergraduate student
Dingtong Tan – Undergraduate student











Cosper Lab Alumni
Maha Paracha, MBBS, MS – Research Specialist

-
SCCA1/SERPINB3 suppresses antitumor immunity and blunts therapy-induced T cell responses via STAT-dependent chemokine production The Journal of clinical investigation
Chen L, Shi V, Wang S, Sun L, Freeman R, Yang J, Inkman MJ, Ghosh S, Ruiz F, Jayachandran K, Huang Y, Luo J, Zhang J, Cosper P, Luke CJ, Spina CS, Grigsby PW, Schwarz JK, Markovina S
2023 Aug 1;133(15):e163841. doi: 10.1172/JCI163841.
-
More
Patients with cancer who have high serum levels of squamous cell carcinoma antigen 1 (SCCA1, now referred to as SERPINB3) commonly experience treatment resistance and have a poor prognosis. Despite being a clinical biomarker, the modulation of SERPINB3 in tumor immunity is poorly understood. We found positive correlations of SERPINB3 with CXCL1, CXCL8 (CXCL8/9), S100A8, and S100A9 (S100A8/A9) myeloid cell infiltration through RNA-Seq analysis of human primary cervical tumors. Induction of SERPINB3 resulted in increased CXCL1/8 and S100A8/A9 expression, which promoted monocyte and myeloid-derived suppressor cell (MDSC) migration in vitro. In mouse models, Serpinb3a tumors showed increased MDSC and tumor-associated macrophage (TAM) infiltration, contributing to T cell inhibition, and this was further augmented upon radiation. Intratumoral knockdown (KD) of Serpinb3a resulted in tumor growth inhibition and reduced CXCL1 and S100A8/A expression and MDSC and M2 macrophage infiltration. These changes led to enhanced cytotoxic T cell function and sensitized tumors to radiotherapy (RT). We further revealed that SERPINB3 promoted STAT-dependent expression of chemokines, whereby inhibition of STAT activation by ruxolitinib or siRNA abrogated CXCL1/8 and S100A8/ A9 expression in SERPINB3 cells. Patients with elevated pretreatment SCCA levels and high phosphorylated STAT3 (p-STAT3) had increased intratumoral CD11b+ myeloid cells compared with patients with low SCCA levels and p-STAT3, who had improved overall survival after RT. These findings provide a preclinical rationale for targeting SERPINB3 in tumors to counteract immunosuppression and improve the response to RT.
PMID:37279067 | PMC:PMC10378164 | DOI:10.1172/JCI163841
View details for PubMedID 37279067
-
More
-
Biological Characterization of the Effects of Filtration on the Xoft Axxent® Electronic Brachytherapy Source for Cervical Cancer Applications Radiation research
Walter AE, Cosper PF, Nickel KP, Ramesh S, Khan AU, DeWerd LA, Kimple RJ
2023 May 1;199(5):429-438. doi: 10.1667/RADE-22-00112.1.
-
More
Low-energy X-ray sources that operate in the kilovoltage energy range have been shown to induce more cellular damage when compared to their megavoltage counterparts. However, low-energy X-ray sources are more susceptible to the effects of filtration on the beam spectrum. This work sought to characterize the biological effects of the Xoft Axxent® source, a low-energy therapeutic X-ray source, both with and without the titanium vaginal applicator in place. It was hypothesized that there would be an increase in relative biological effectiveness (RBE) of the Axxent® source compared to 60Co and that the source in the titanium vaginal applicator (SIA) would have decreased biological effects compared to the bare source (BS). This hypothesis was drawn from linear energy transfer (LET) simulations performed using the TOPAS Monte Carlo user code as well a reduction in dose rate of the SIA compared to the BS. A HeLa cell line was maintained and used to evaluate these effects. Clonogenic survival assays were performed to evaluate differences in the RBE between the BS and SIA using 60Co as the reference beam quality. Neutral comet assay was used to assess induction of DNA strand damage by each beam to estimate differences in RBE. Quantification of mitotic errors was used to evaluate differences in chromosomal instability (CIN) induced by the three beam qualities. The BS was responsible for the greatest quantity of cell death due to a greater number of DNA double strand breaks (DSB) and CIN observed in the cells. The differences observed in the BS and SIA surviving fractions and RBE values were consistent with the 13% difference in LET as well as the factor of 3.5 reduction in dose rate of the SIA. Results from the comet and CIN assays were consistent with these results as well. The use of the titanium applicator results in a reduction in the biological effects observed with these sources, but still provides an advantage over megavoltage beam qualities. © 2023 by Radiation Research Society.
PMID:37014873 | PMC:PMC10288372 | DOI:10.1667/RADE-22-00112.1
View details for PubMedID 37014873
-
More
-
HPV16 E6 induces chromosomal instability due to polar chromosomes caused by E6AP-dependent degradation of the mitotic kinesin CENP-E Proceedings of the National Academy of Sciences of the United States of America
Cosper PF, Hrycyniak CF, Paracha M, Lee DL, Wan J, Jones K, Bice SA, Nickel K, Mallick S, Taylor AM, Kimple RJ, Lambert PF, Weaver BA
2023 Apr 4;120(14):e2216700120. doi: 10.1073/pnas.2216700120. Epub 2023 Mar 29.
-
More
Chromosome segregation during mitosis is highly regulated to ensure production of genetically identical progeny. Recurrent mitotic errors cause chromosomal instability (CIN), a hallmark of tumors. The E6 and E7 oncoproteins of high-risk human papillomavirus (HPV), which causes cervical, anal, and head and neck cancers (HNC), cause mitotic defects consistent with CIN in models of anogenital cancers, but this has not been studied in the context of HNC. Here, we show that HPV16 induces a specific type of CIN in patient HNC tumors, patient-derived xenografts, and cell lines, which is due to defects in chromosome congression. These defects are specifically induced by the HPV16 oncogene E6 rather than E7. We show that HPV16 E6 expression causes degradation of the mitotic kinesin CENP-E, whose depletion produces chromosomes that are chronically misaligned near spindle poles (polar chromosomes) and fail to congress. Though the canonical oncogenic role of E6 is the degradation of the tumor suppressor p53, CENP-E degradation and polar chromosomes occur independently of p53. Instead, E6 directs CENP-E degradation in a proteasome-dependent manner via the E6-associated ubiquitin protein ligase E6AP/UBE3A. This study reveals a mechanism by which HPV induces CIN, which may impact HPV-mediated tumor initiation, progression, and therapeutic response.
PMID:36989302 | PMC:PMC10083562 | DOI:10.1073/pnas.2216700120
View details for PubMedID 36989302
-
More
-
SCCA1/SERPINB3 promotes suppressive immune environment via STAT-dependent chemokine production, blunting the therapy-induced T cell responses bioRxiv : the preprint server for biology
Chen L, Shi V, Wang S, Freeman R, Ruiz F, Jayachandran K, Zhang J, Cosper P, Sun L, Luke CJ, Spina C, Grigsby PW, Schwarz JK, Markovina S
2023 Feb 3:2023.02.01.526675. doi: 10.1101/2023.02.01.526675.
-
More
Radiotherapy is a commonly used cancer treatment; however, patients with high serum squamous cell carcinoma antigen (SCCA1/SERPINB3) are associated with resistance and poor prognosis. Despite being a strong clinical biomarker, the modulation of SERPINB3 in tumor immunity is poorly understood. We investigated the microenvironment of SERPINB3 high tumors through RNAseq of primary cervix tumors and found that SERPINB3 was positively correlated with CXCL1/8, S100A8/A9 and myeloid cell infiltration. Induction of SERPINB3 in vitro resulted in increased CXCL1/8 and S100A8/A9 production, and supernatants from SERPINB3-expressing cultures attracted monocytes and MDSCs. In murine tumors, the orthologue mSerpinB3a promoted MDSC, TAM, and M2 macrophage infiltration contributing to an immunosuppressive phenotype, which was further augmented upon radiation. Radiation-enhanced T cell response was muted in SERPINB3 tumors, whereas Treg expansion was observed. A STAT-dependent mechanism was implicated, whereby inhibiting STAT signaling with ruxolitinib abrogated suppressive chemokine production. Patients with elevated pre-treatment serum SCCA and high pSTAT3 had increased intratumoral CD11b+ myeloid cell compared to patients with low SCCA and pSTAT3 cohort that had overall improved cancer specific survival after radiotherapy. These findings provide a preclinical rationale for targeting STAT signaling in tumors with high SERPINB3 to counteract the immunosuppressive microenvironment and improve response to radiation.
PMID:36778224 | PMC:PMC9915608 | DOI:10.1101/2023.02.01.526675
View details for PubMedID 36778224
-
More
-
Chromosome Missegregation as a Modulator of Radiation Sensitivity Seminars in radiation oncology
Cosper PF, Copeland SE, Tucker JB, Weaver BA
2022 Jan;32(1):54-63. doi: 10.1016/j.semradonc.2021.09.002.
-
More
Chromosome missegregation over the course of multiple cell divisions, termed chromosomal instability (CIN), is a hallmark of cancer. Multiple causes of CIN have been identified, including defects in the mitotic checkpoint, altered kinetochore-microtubule dynamics, centrosome amplification, and ionizing radiation. Here we review the types, mechanisms, and cellular implications of CIN. We discuss the evidence that CIN can promote tumors, suppress them, or do neither, depending on the rates of chromosome missegregration and the cellular context. Very high rates of chromosome missegregation lead to cell death due to loss of essential chromosomes; thus elevating CIN above a tolerable threshold provides a mechanistic opportunity to promote cancer cell death. Lethal rates of CIN can be achieved by a single insult or through a combination of insults. Because ionizing radiation induces CIN, additional therapies that increase CIN may serve as useful modulators of radiation sensitivity. Ultimately, quantifying the intrinsic CIN in a tumor and modulating this level pharmacologically as well as with radiation may allow for a more rational, personalized radiation therapy prescription, thereby decreasing side effects and increasing local control.
PMID:34861996 | PMC:PMC8883596 | DOI:10.1016/j.semradonc.2021.09.002
View details for PubMedID 34861996
-
More
-
Why De-Intensification is not Possible in HPV-Associated Cervical Cancer Seminars in radiation oncology
Beaty BT, Cosper PF, Beriwal S, Weiner AA
2021 Oct;31(4):339-348. doi: 10.1016/j.semradonc.2021.06.001.
-
Biology of HPV Mediated Carcinogenesis and Tumor Progression Seminars in radiation oncology
Cosper PF, Bradley S, Luo L, Kimple RJ
2021 Oct;31(4):265-273. doi: 10.1016/j.semradonc.2021.02.006.
-
More
Human papillomavirus (HPV) is a ubiquitous DNA virus that infects squamous epithelia. Though HPV only encodes 8 genes, it is capable of causing cellular transformation and ultimately cancer in host cells. In this article we review the classification of HPV viruses, their genetic structure and life cycle, viral gene biology, and provide an overview of the role of HPV in cancer. We explain how the viral life cycle can lead to integration of viral DNA into the host genome leading to increased cell cycle progression, decreased apoptosis, altered DNA repair, and chromosomal instability. We describe the multifaceted roles of the canonical oncogenes E6 and E7 in promoting tumorigenesis and the important role of other viral genes in regulating cancer development. We also review how the virus actively suppresses innate and adaptive immunity to evade immune detection and promote a pro-tumorigenic microenvironment. The biology presented here will serve as a foundation to the other chapters in this edition and we hope it will incite enthusiasm for continued research on this fascinating virus that causes significant morbidity and mortality worldwide.
PMID:34455982 | PMC:PMC8409095 | DOI:10.1016/j.semradonc.2021.02.006
View details for PubMedID 34455982
-
More
-
Reply to A. J. Cmelak et al and B. Kalra et al Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Chundury A, Cosper PF, Kim S
2021 Aug 20;39(24):2735-2736. doi: 10.1200/JCO.21.00885. Epub 2021 May 27.
-
Localized Delivery of Cisplatin to Cervical Cancer Improves Its Therapeutic Efficacy and Minimizes Its Side Effect Profile International journal of radiation oncology, biology, physics
Federico C, Sun J, Muz B, Alhallak K, Cosper PF, Muhammad N, Jeske A, Hinger A, Markovina S, Grigsby P, Schwarz JK, Azab AK
2021 Apr 1;109(5):1483-1494. doi: 10.1016/j.ijrobp.2020.11.052. Epub 2020 Nov 27.
-
More
PURPOSE: Cervical cancer represents the fourth most frequent malignancy in the world among women, and mortality has remained stable for the past 4 decades. Intravenous cisplatin with concurrent radiation therapy is the standard-of-care for patients with local and regional cervical cancer. However, cisplatin induces serious dose-limiting systemic toxicities and recurrence frequently occurs. In this study, we aimed to develop an intracervical drug delivery system that allows cisplatin release directly into the tumor and minimize systemic side effects.
METHODS AND MATERIALS: Twenty patient biopsies and 5 cell lines treated with cisplatin were analyzed for platinum content using inductively coupled plasma mass spectrometry. Polymeric implants loaded with cisplatin were developed and evaluated for degradation and drug release. The effect of local or systemic cisplatin delivery on drug biodistribution as well as tumor burden were evaluated in vivo, in combination with radiation therapy.
RESULTS: Platinum levels in patient biopsies were 6-fold lower than the levels needed for efficacy and radiosensitization in vitro. Cisplatin local delivery implant remarkably improved drug specificity to the tumor and significantly decreased accumulation in the blood, kidney, and other distant normal organs, compared with traditional systemic delivery. The localized treatment further resulted in complete inhibition of tumor growth.
CONCLUSIONS: The current standard-of-care systemic administration of cisplatin provides a subtherapeutic dose. We developed a polymeric drug delivery system that delivered high doses of cisplatin directly into the cervical tumor, while lowering drug accumulation and consequent side effects in normal tissues. Moving forward, these data will be used as the basis of a future first-in-human clinical trial to test the efficacy of localized cisplatin as adjuvant or neoadjuvant chemotherapy in local and regional cervical cancer.
PMID:33253820 | PMC:PMC8592040 | DOI:10.1016/j.ijrobp.2020.11.052
View details for PubMedID 33253820
-
More
-
Patient Derived Models to Study Head and Neck Cancer Radiation Response Cancers
Cosper PF, Abel L, Lee Y, Paz C, Kaushik S, Nickel KP, Alexandridis R, Scott JG, Bruce JY, Kimple RJ
2020 Feb 12;12(2):419. doi: 10.3390/cancers12020419.
-
More
Patient-derived model systems are important tools for studying novel anti-cancer therapies. Patient-derived xenografts (PDXs) have gained favor over the last 10 years as newer mouse strains have improved the success rate of establishing PDXs from patient biopsies. PDXs can be engrafted from head and neck cancer (HNC) samples across a wide range of cancer stages, retain the genetic features of their human source, and can be treated with both chemotherapy and radiation, allowing for clinically relevant studies. Not only do PDXs allow for the study of patient tissues in an in vivo model, they can also provide a renewable source of cancer cells for organoid cultures. Herein, we review the uses of HNC patient-derived models for radiation research, including approaches to establishing both orthotopic and heterotopic PDXs, approaches and potential pitfalls to delivering chemotherapy and radiation to these animal models, biological advantages and limitations, and alternatives to animal studies that still use patient-derived tissues.
PMID:32059418 | PMC:PMC7072508 | DOI:10.3390/cancers12020419
View details for PubMedID 32059418
-
More
-
Decreased local immune response and retained HPV gene expression during chemoradiotherapy are associated with treatment resistance and death from cervical cancer International journal of cancer
Cosper PF, McNair C, González I, Wong N, Knudsen KE, Chen JJ, Markovina S, Schwarz JK, Grigsby PW, Wang X
2020 Apr 1;146(7):2047-2058. doi: 10.1002/ijc.32793. Epub 2019 Dec 4.
-
More
More than one-third of patients with locally advanced cervical cancer do not respond to chemoradiation therapy (CRT). We aimed to characterize the transcriptional landscape of paired human cervical tumors before and during CRT in order to gain insight into the evolution of treatment response and to elucidate mechanisms of treatment resistance. We prospectively collected cervical tumor biopsies from 115 patients both before and 3 weeks into CRT. RNA-sequencing, Gene Set Enrichment Analysis and HPV gene expression were performed on 20 paired samples that had adequate neoplastic tissue mid-treatment. Tumors from patients with no evidence of disease (NED) at last follow-up had enrichment in pathways related to the immune response both pretreatment and mid-treatment, while tumors from patients dead of disease (DOD) demonstrated enrichment in biosynthetic and mitotic pathways but not in immune-related pathways. Patients DOD had decreased expression of T-cell and cytolytic genes and increased expression of PD-L2 mid-treatment compared to patients NED. Histological and immunohistochemical analysis revealed a decrease in tumor-associated lymphocytes (TAL) during CRT in all patients but tumors from patients DOD had a significantly more pronounced decrease in TALs and CD8+ cells mid-treatment, which was validated in a larger mid-treatment cohort. Finally, patients DOD retained more HPV E6/E7 gene expression during CRT and this was associated with increased expression of genes driving mitosis, which was corroborated in vitro. Our results suggest that decreased local immune response and retained HPV gene expression may be acting together to promote treatment resistance during CRT in patients with cervical cancer.
PMID:31732968 | PMC:PMC8177718 | DOI:10.1002/ijc.32793
View details for PubMedID 31732968
-
More
-
Intensity modulated radiation therapy and surgery for Management of Retroperitoneal Sarcomas: a single-institution experience Radiation oncology (London, England)
Cosper PF, Olsen J, DeWees T, Van Tine A, Hawkins W, Michalski J, Zoberi I
2017 Dec 8;12(1):198. doi: 10.1186/s13014-017-0920-y.
-
More
BACKGROUND: Peri-operative radiation of retroperitoneal sarcomas (RPS) is an important component of multidisciplinary treatment. All retrospective series thus far included patients treated with older radiation therapy (RT) techniques including 2D and 3DRT. Intensity modulated radiation therapy (IMRT) allows for selective dose escalation while sparing adjacent organs. We therefore report the first series of patients with RPS treated solely with IMRT, surgery and chemotherapy. We hypothesized that IMRT would permit safe dose escalation and superior rates of local control (LC) in this high-risk patient population.
METHODS: Thirty patients with RPS treated with curative intent between 2006 and 2015 were included in this retrospective study. RT was administered either pre- or post-operatively and IMRT was used in all patients. Statistical comparisons, LC, distant metastasis (DM), and overall survival (OS) were calculated by Kaplan-Meier analysis and univariate Cox regression.
RESULTS: Median follow-up time after completion of RT was 36 months (range 1.4-112). Median tumor size was 14 cm (range 3.6 - 28 cm). The most prevalent histologies were liposarcoma in 10 (33%) patients and leiomyosarcoma in 10 (33%) with 21 patients (70%) having high-grade disease. Twenty-eight (93%) patients had surgical resection with 47% having positive margins. Chemotherapy was administered in 9 (30%) patients. RT was delivered pre-operatively in 11 (37%) patients, and post-operatively in 19 (63%) with 60% of patients receiving a simultaneous integrated boost. Pre-operative median RT dose to the high-risk area was 55 Gy (range, 43-66 Gy) while median post-operative dose was 60.4 Gy (range, 45-66.6 Gy). There was one acute grade 3 and one late grade 3 toxicity and no grade 4 or 5 toxicities. Three year actuarial LC, freedom from DM, and OS rates were 84%, 64%, and 68% respectively. Positive surgical margins were associated with a higher risk of local recurrence (p = 0.02) and decreased OS (p = 0.04). Pre-operative RT was associated with improved LC (p = 0.1) with a 5-year actuarial LC of 100%. Administration of chemotherapy, timing of RT, histology or grade was not predictive of OS.
CONCLUSIONS: Patients with RPS treated with peri-operative IMRT at our institution had excellent local control and low incidences of toxicity.
PMID:29216884 | PMC:PMC5721605 | DOI:10.1186/s13014-017-0920-y
View details for PubMedID 29216884
-
More
-
Neoadjuvant Ifosfamide and Epirubicin in the Treatment of Malignant Peripheral Nerve Sheath Tumors Sarcoma
Hirbe AC, Cosper PF, Dahiya S, Van Tine A
2017;2017:3761292. doi: 10.1155/2017/3761292. Epub 2017 May 4.
-
More
Background and Objectives. Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive soft tissue sarcomas with poor overall survival. Response to chemotherapy has been debated for these tumors. Methods. We performed a retrospective analysis of the patients at our institution with a biopsy-proven diagnosis of MPNST that underwent neoadjuvant chemotherapy prior to surgery. Results. We retrospectively identified five patients who received neoadjuvant chemotherapy with epirubicin and ifosfamide that demonstrated a 30% reduction in tumor growth and a 60% response rate by RECIST criteria. Additionally, a metabolic response was observed in all three patients who received serial PET scans during neoadjuvant treatment. The clinical benefit rate, which includes stable disease, was 100%. Conclusions. Our data suggest that MPNSTs do respond to epirubicin and ifosfamide based chemotherapy and prospective studies are warranted to further define the clinical benefit.
PMID:28546782 | PMC:PMC5435903 | DOI:10.1155/2017/3761292
View details for PubMedID 28546782
-
More
-
Improving Clinical Alarm Management: Guidance and Strategies Biomedical instrumentation & technology
Cosper P, Zellinger M, Enebo A, Jacques S, Razzano L, Flack MN
2017 Mar-Apr;51(2):109-115. doi: 10.2345/0899-8205-51.2.109.
-
More
This article focuses on the type of problems that lead to false or nonactionable clinical alarms and the type of data that can help identify which of these alarms are most prevalent in specific units in healthcare facilities. The process of identifying necessary data is first described, as this activity will drive later choices on capturing data. This article also discusses how to use the data collected in alarm reports to help determine which alarms should be targeted first for improved management in a pilot environment. Suggestions are provided on how to reduce false and nonactionable alarm signals and how to monitor to ensure no untoward consequences occur from new alarm default settings. The information provided here can be individualized to hospitals and units to enhance alarm management with physiological monitor alarms. It also can be adapted to reduce nonactionable alarm signals occurring from other medical devices.
PMID:28296432 | DOI:10.2345/0899-8205-51.2.109
View details for PubMedID 28296432
-
More
-
Prospective analysis of toxicity in patients treated with strut-adjusted volume implant for early-stage breast cancer Brachytherapy
Rehman S, Agarwal R, Ochoa L, Cosper P, Zoberi J, Cyr A, Margenthaler J, Thomas M, Zoberi I
2016 Sep-Oct;15(5):625-30. doi: 10.1016/j.brachy.2016.04.008. Epub 2016 Jun 1.
-
More
PURPOSE: We report the toxicity of patients treated with strut-adjusted volume implant (SAVI) for accelerated partial breast irradiation treated at our institution.
METHODS AND MATERIALS: Patients treated from January 2013 to July 2015 with SAVI planned for 10 b.i.d. fractions for a total dose of 34 Gy were included. Acute and late toxicities were prospectively collected on patients in followup and graded by the Common Terminology Criteria for Adverse Events, version 4.0.
RESULTS: A total of 132 patients were included, with 1 patient having synchronous breast cancer treated in each breast. Median followup was 20.0 months (range, 2.7-37.4 months). The median age at diagnosis was 61 years (range, 41-83 years). Forty-two lesions (32%) were in situ, 88 lesions (66%) were Stage 1, and 3 (2%) lesions were Stage 2. The median planning target volume was 58.2 cc (range, 24.2-109.9 cc), median V150 was 26.3 cc (range, 11.5-47.5 cc), and median V200 was 13.0 cc (range, 6.3-26.1 cc). On a pain scale of 0-10 (10 = worst pain), pain was worst on Day 2 of treatment, with an average score of 0.46. There was one acute skin infection; there were three late skin infections, two of which was Grade 3. Other late toxicities were Grade 1 or 2: hyperpigmentation (44%), telangiectasia (0.8%), seroma (9%), fat necrosis (5%), and fibrosis (12%). Crude local recurrence rate was 4%.
CONCLUSION: SAVI is a safe treatment option for patients who are candidates for accelerated partial breast irradiation. Local control seems to be excellent, but longer followup is needed.
PMID:27263058 | DOI:10.1016/j.brachy.2016.04.008
View details for PubMedID 27263058
-
More
-
Myosin heavy chain is not selectively decreased in murine cancer cachexia International journal of cancer
Cosper PF, Leinwand LA
2012 Jun 1;130(11):2722-7. doi: 10.1002/ijc.26298. Epub 2011 Aug 30.
-
More
Cachexia is a severe muscle-wasting syndrome associated with several chronic diseases such as cancer and AIDS. Muscle mass loss significantly decreases prognosis and survival. The mechanisms of muscle atrophy and the specific proteins targeted for degradation have been intensely studied and are potential therapeutic targets. Published reports that myosin heavy chain (MyHC), the most abundant protein by mass in skeletal muscle, is selectively targeted for degradation in cancer cachexia remain controversial. Here we show that the results of previous studies showing a selective decrease in MyHC are likely an artifact resulting from muscle lysis methods which do not solubilize myosin out of myofibrils. We show that MyHC decreases in parallel with other myofibrillar proteins in cachectic skeletal muscle, which has mechanistic and therapeutic implications. These findings should lead to mechanistic insight into the stoichiometry of sarcomeric disassembly and degradation during cancer cachexia.
PMID:21796617 | PMC:PMC3267878 | DOI:10.1002/ijc.26298
View details for PubMedID 21796617
-
More
-
Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner Cancer research
Cosper PF, Leinwand LA
2011 Mar 1;71(5):1710-20. doi: 10.1158/0008-5472.CAN-10-3145. Epub 2010 Dec 16.
-
More
Approximately one-third of cancer deaths are caused by cachexia, a severe form of skeletal muscle and adipose tissue wasting that affects men more than women. The heart also undergoes atrophy in cancer patients, but the mechanisms and the basis for apparent sex differences are unclear. In a mouse colon-adenocarcinoma model, cancer causes a loss of cardiac mass due to a decrease in cardiac myocyte size that is associated with reduced levels of all sarcomeric proteins. Unlike skeletal muscle cachexia, atrophic hearts do not upregulate the ubiquitin-proteasome system or its activity but increase autophagy. Thus, cancer causes cardiac atrophy by a mechanism distinct from that in skeletal muscle. Male tumor-bearing mice have a more severe phenotype than females, including greater cardiac mass loss and mortality, a more robust pro-inflammatory response to the tumor, and greater cardiac autophagy. In females, estrogen protects against cancer-induced cardiac atrophy and body weight loss by signaling through its receptor. Sex differences in cardiac atrophy need to be considered during the treatment of patients suffering from chemotherapy-induced cardiomyopathy to prevent exacerbation of cardiac dysfunction.
PMID:21163868 | PMC:PMC3049989 | DOI:10.1158/0008-5472.CAN-10-3145
View details for PubMedID 21163868
-
More
-
Cytokines differentially regulate the synthesis of prostanoid and nitric oxide mediators in tumorigenic versus non-tumorigenic mouse lung epithelial cell lines Carcinogenesis
Dwyer-Nield LD, Srebernak MC, Barrett BS, Ahn J, Cosper P, Meyer AM, Kisley LR, Bauer AK, Thompson DC, Malkinson AM
2005 Jul;26(7):1196-206. doi: 10.1093/carcin/bgi061. Epub 2005 Mar 3.
-
More
Studies using transgenic and knockout mice have demonstrated that particular cytokines influence lung tumor growth and identified prostaglandin E2 (PGE2), prostacyclin (PGI2) and nitric oxide (NO) as critical mediators of this process. PGE2 and NO were pro-tumorigenic while PGI2 was antitumorigenic. We describe herein an in vitro experimental approach to examine interactions among cytokines, prostaglandins (PGs) and NO. PGE2, PGI2, and NO levels were assayed in culture media from non-tumorigenic mouse lung epithelial cell lines, their spontaneous transformants and mouse lung tumor-derived cell lines, before or after exposure to the cytokines TNFalpha, IFNgamma and IL1beta, alone and in combination. More PGE2 than PGI2 was produced by neoplastic cells, while the opposite was observed in non-tumorigenic lines. Cytokine exposure magnified the extent of these differential concentrations. The PGE2 to PGI2 ratio was also greater in chemically-induced mouse lung tumors than in adjacent tissue or control lungs, supporting the physiological relevance of this in vitro model. Expression of PG biosynthetic enzymes in these cell lines correlated with production of the corresponding PGs. Cytokine treatment enhanced NO production by inducing the inflammation-associated biosynthetic enzyme, inducible NO synthase (iNOS), but this did not correlate with the neoplastic status of cells. Inhibition of iNOS or cyclooxygenase 2 activity using aminoguanidine or NS-398 respectively, demonstrated that NO did not affect PG production nor did PGs influence NO production. Since lack of iNOS inhibits mouse lung tumor formation, we propose that this is independent of any modulation of PG synthesis in epithelial cells. The similar normal/neoplastic trends in PGE2 to PGI2 ratios both in vitro and in vivo, together with an amplification of this difference upon cytokine exposure, are consistent with the hypothesis that cytokines released during inflammation exacerbate differences in the behavior of neoplastic and normal lung cells.
PMID:15746162 | DOI:10.1093/carcin/bgi061
View details for PubMedID 15746162
-
More
-
Relative amounts of antagonistic splicing factors, hnRNP A1 and ASF/SF2, change during neoplastic lung growth: implications for pre-mRNA processing Molecular carcinogenesis
Zerbe LK, Pino I, Pio R, Cosper PF, Dwyer-Nield LD, Meyer AM, Port JD, Montuenga LM, Malkinson AM
2004 Dec;41(4):187-96. doi: 10.1002/mc.20053.
-
More
Pre-mRNA processing is an important mechanism for globally modifying cellular protein composition during tumorigenesis. To understand this process during lung cancer, expression of two key pre-mRNA alternative splicing factors was compared in a mouse model of early lung carcinogenesis and during regenerative growth following reversible lung injury. Heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and alternative splicing factor/splicing factor 2 (ASF/SF2) act antagonistically to modulate splice site selection. Both hnRNP A1 and ASF/SF2 contents rose in adenomas and during injury-induced hyperplasia compared to control lungs, as measured by immunoblotting. While both proteins increased similarly during compensatory hyperplasia, hnRNP A1 increased to a much greater extent than ASF/SF2 in tumors, resulting in a 6-fold increase of the hnRNP A1 to ASF/SF2 ratio. Immunohistochemical analysis showed that hnRNP A1 localized exclusively within tumor nuclei, while ASF/SF2 appeared in cytoplasm and/or nuclei, depending on the growth pattern of the tumor cells. We also demonstrated cancer-associated changes in the pre-mRNA alternative splicing of CD44, a membrane glycoprotein involved in cell-cell and cell-extracellular matrix interactions. hnRNP A1 and ASF/SF2 expression is thus differentially altered in neoplastic lung cells by mechanisms that do not strictly arise from increased cell division. These changes are influenced by tumor histology and may be associated with production of variant CD44 mRNA isoforms.
PMID:15390079 | DOI:10.1002/mc.20053
View details for PubMedID 15390079
-
More
-
Renal cancer complicating acquired cystic kidney disease Journal of the American Society of Nephrology : JASN
Gupta A, Cosper PC
1994 Dec;5(6):1407-8.
Contact Information
Pippa Cosper, MD, PhD
1111 Highland Avenue,WIMR I 3057
Madison, WI 53703
