I am a tenured faculty in the Department of Human Oncology with a clinical practice and translational research laboratory. I specialize in treating patients with cancers of the head and neck. I am a member of the UW Multidisciplinary Head and Neck Program and work closely with head and neck surgeons, medical oncologists, radiologists, speech and swallow therapists and other specialists to best meet the individual patient’s needs.
The goal of my lab is to improve the care of cancer patients through translational cancer research while providing a supportive world-class learning environment for scientists at all levels and from all backgrounds. We pursue this goal by studying the molecular mechanisms underlying the response to radiation and the development of therapeutic resistance in head and neck and lung cancer and by developing approaches to treat side effects of radiation therapy. We utilize cellular and mouse models that we have developed including patient-derived xenografts and intrinsic resistance models to understand therapeutic response. More recently, we have developed cellular therapies we are using to reverse radiation-induced damage to the salivary glands.
In addition to my clinical and research roles, I teach undergraduate, graduate and medical students as well as residents and postdoctoral fellows. I try to give these students a sense of the breadth of opportunities in medicine and challenge them to be the best doctors and scientists they can be. I encourage my students to come to the clinic with me so that they can see cancer from our patient’s perspective.
Dr. Kimple's UW Health ProfileEducation
MBA, Edgewood College, (2022 - 2023)
Postdoctoral Fellow, University of Wisconsin–Madison, Tumor Virology (2010 - 2012)
Postdoctoral Fellow, University of North Carolina at Chapel Hill, Cancer Biology (2008 - 2010)
Resident, University of North Carolina at Chapel Hill, Radiation Oncology (2006 - 2010)
Intern, University of North Carolina at Chapel Hill, Internal Medicine (2005 - 2006)
MD, University of North Carolina at Chapel Hill, Medicine (1998 - 2005)
PhD, University of North Carolina at Chapel Hill, Pharmacology (2000 - 2003)
BS, Michigan State University, . Environmental Science and Management (1994 - 1998)
Academic Appointments
Professor with tenure, Department of Human Oncology (2024 - present)
Professor, Affiliate, Department of Medical Physics (2024 - present)
Member , UW Stem Cell and Regenerative Medicine Center (2023 - present)
Member, Center for Human Genomics and Precision Medicine (2022 - present)
Faculty Director, Graduate Program in Clinical Investigation (2020 - present)
Associate Professor with tenure, Human Oncology (2018 - 2024)
Associate Professor, Affiliate, Department of Medical Physics (2018 - 2024)
Co-Leader, Imaging and Radiation Sciences Program, UW Carbone Cancer Center (2018 - present)
Director, Cancer Biology and Translational Medicine Division, Department of Human Oncology (2018 - present)
Assistant Professor, Human Oncology (2012 - 2018)
Assistant Professor, Medical Physics (2014 - 2018)
Member, UW Carbone Cancer Center (2012 - present)
Member, UW Institute for Clinical and Translational Research (2011 - present)
Selected Honors and Awards
Fellow of American Society for Radiation Oncology (ASTRO) (2022)
Association of Residents in Radiation Oncology Teacher of Year Award (2017)
University of Wisconsin Postdoctoral Mentor Award (2017)
Medical Faculty Award, University of North Carolina (2005)
Lineberger Comprehensive Cancer Center Graduate Fellow Award (2002)
Outstanding Senior Award, Michigan State University (1998)
College of Natural Science Convocation Speaker, Michigan State University (1998)
Tower Guard Sophomore Honor Service Society, Michigan State University (1995-1996)
Boards, Advisory Committees and Professional Organizations
American Society for Pharmacology and Experimental Therapeutics (2003 - present)
Radiological Society of North America (2006 - present)
American Society for Radiation Oncology (2006 - present)
American Society for Clinical Oncology (2006 - present)
University of Wisconsin Institute for Clinical and Translational Research (2010 - present)
Radiation Research Society (2012 - present)
American Society of Clinical Oncology Leadership Development Program (2017 - 2018)
Co-chair, Big Ten Cancer Research Consortium Head and Neck Working Group (2017 - 2021)
ASTRO Annual Meeting Scientific Track co-chair – Biology (2017 - 2022)
ASTRO Annual Meeting Scientific Track chair – Biology (2019 - 2022)
Radiation Oncology Institute Research Committee (2019 - present)
International Journal of Radiation Oncology Biology and Physics, Senior Associate Editor and Senior Associate Editor (2014 - 2020)
Vice-Chair, RSNA R&E Radiation Oncology Research Study Section (2019 - 2021)
Chair, RSNA R&E Radiation Oncology Research Study Section (2021 - 2024)
International Journal of Radiation Oncology Biology and Physics, Critical Reviews Editor (2021 - present)
Head and Neck Work Group, Alliance Experimental Therapeutics and Rare Tumor Committee, Member (2020 - present)
Metastatic-Recurrent Task Force, NCI Head and Neck Cancer Steering Committee, member (2021 - 2027)
Chair, ASTRO Advancing Research Talent Committee (2021 - 2023)
ASTRO Science Council Steering Committee, vice chair (2023 - present)
Research Focus
Head & Neck Cancer
Dr. Randall Kimple specializes in treating patients with malignancies of the head and neck. In his research laboratory, he uses patient-derived xenografts to test radiation, chemotherapy and combinations of therapies to understand which characteristics of a patient’s tumor may predict response to treatment.
Kimple Lab

Mission Statement
The mission of our lab is to cultivate an inclusive and supportive environment where trainees at all levels develop the skills to effectively question, research, and communicate scientific topics. We recognize that our members come from diverse backgrounds, with varied experiences, perspectives, and career goals. We are committed to fostering a respectful and equitable community where everyone feels empowered to contribute and succeed, while providing individualized mentorship and opportunities that align with their unique needs and aspirations.
Vision Statement
Our vision is a future where cancer patients experience improved outcomes and reduced treatment toxicity. We strive to achieve this by fostering a collaborative and inclusive environment where our trainees develop into exceptional scientists, equipped to make significant contributions to the fight against cancer and improve the lives of patients.
Lab Values & Expectations
- Embrace challenges and learn to persevere
- Cultivate intellectual curiosity
- Pursue rigorous and reproducible research
- Communicate effectively
- Take care of yourself and others
- Treat everyone with respect and courtesy
- Have fun
Big Picture
Radiation therapy can be used to cure many cancer patients. We use powerful patient-derived model systems to study how cancers evolute to overcome current treatments and study treatments to overcome radiation-induced toxicity to improve the quality of life of our patients. Our long-term goal is to offer personalized treatments to each patient.
1) Cell therapies for radiation toxicity
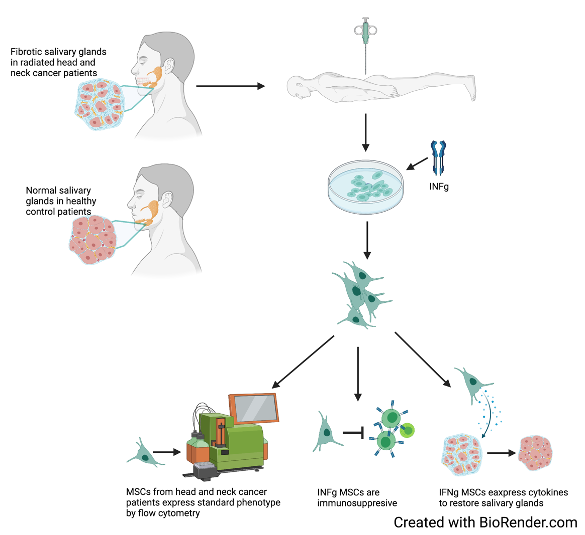
Radiation-induced xerostomia (RIX) represents one of the most common long-term side effects seen in patients who receive radiotherapy to the head and neck. Head and neck cancer (HNC) accounts for nearly 4% of cancers within the United States, estimates show that over 60,000 new cases with occur in 2021. Most HNC treatments include radiation therapy (RT), chemotherapy, targeted therapy, surgery, or a combination of treatments. RT is a primary treatment modality in HNC, while effective in treating cancer, RT causes auxiliary damage to surrounding normal tissues. Xerostomia is a subjective condition of dry mouth hallmarked by decreased saliva production and/or alterations in the composition of saliva. Radiation impairs the saliva-producing acinar cells along with inducing functional changes in the salivary gland ultimately leading to xerostomia. RIX adversely affects quality of life in patients, aside from the general discomfort of dry mouth, patients experience increased dental carries, alterations in taste, fissures in and around the mouth, difficulty swallowing, chewing, and speaking. Currently, there are no available treatment options that address the causes of xerostomia, existing options for patients suffering from RIX are either palliative or accompanied by harsh side effects. There is a critical need to develop safe and effective therapies for RIX. Emerging preclinical and clinical research suggests administration of mesenchymal stromal cells (MSC) to the radiation-damaged salivary gland can lead to increased saliva production and rescue of glandular structures. While it has been demonstrated that MSCs benefit the radiation-damaged salivary gland there is little showing the mechanisms behind MSC function within the gland. The goal of my project is to elucidate how MSCs benefit the radiation-damaged salivary gland in a murine model. Ultimately, the identification of a mechanism will lead to the development of novel, effective, and safe treatment option for RIX.
Funding
- NIH/NIDCR UG3 DE030431
Relevant publications
https://pubmed.ncbi.nlm.nih.gov/?term=kimple+rj+and+MSC&sort=pubdate
2) Patient Derived Model Systems
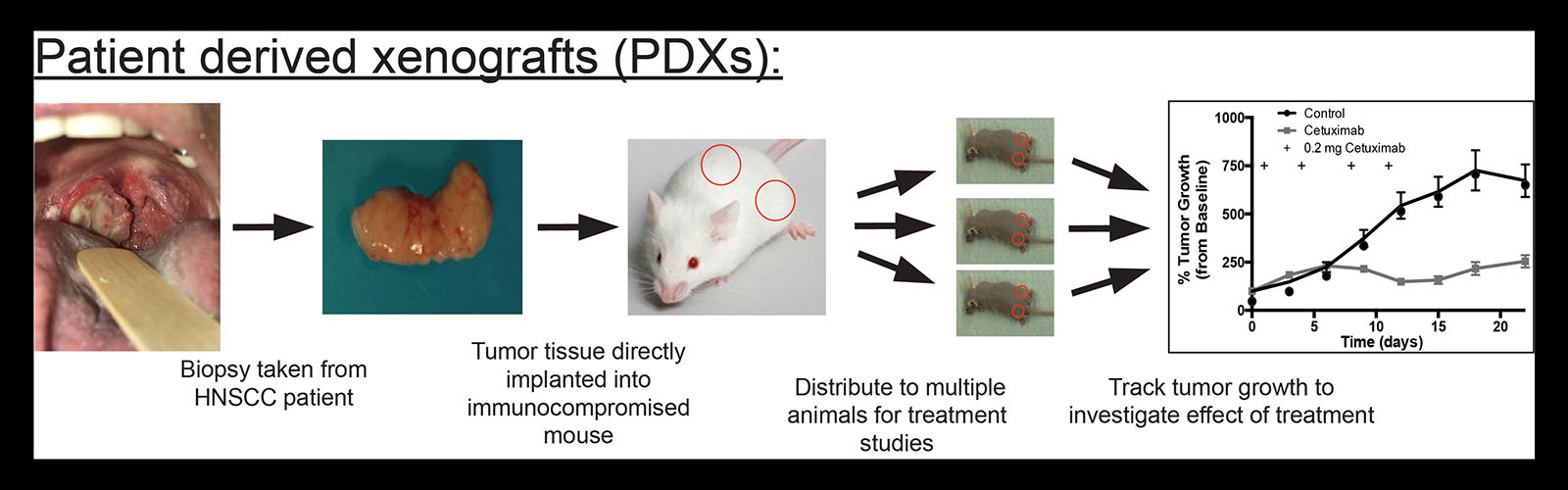
We have established one of the largest tissue repositories of head and neck cancer patient-derived xenografts and have helped define best practices for the establishment, passage, and use of these valuable resources. Patient derived xenografts are established in mice directly from patient biopsies and are thought to better represent the biology of their human source than model cell lines grown in plastic tissue culture plates. Current work seeks to understand how the decisions we make when we establish these models influences their use as personal avatars and drives tumor evolution. We use single-cell approaches to study changes in the tumor’s gene expression, genome, and proteome. In addition, we are beginning to utilize “humanized” models in which the mouse host has a functional immune system. This would allow us to use this resource to study immunotherapy and immunomodulation.
In addition to our focus on head and neck cancer models, we have also established PDXs from brain metastases in lung cancer patients, pancreas cancer, melanoma, breast cancer, and from rare tumors such as adenoid cystic carcinoma and NUT midline carcinomas. We have used our PDXs to partner with several pharmaceutical companies to test novel drugs in head and neck cancers. Please contact us if you have interest in this aspect of our work.
Relevant publications:
https://pubmed.ncbi.nlm.nih.gov/?term=Kimple+RJ+and+%28PDX+or+tumorgraft%29&sort=date
3) Modification of Radiation Response and Improving Delivery of Radiation
We have used the patient-derived systems we have developed to study radiation sensitizers and understand the impact of molecularly targeted agents on radiation response in oncogene driven cancers (SenthilKumar Mol Cancer Ther 2020, Fisher Int J Rad Bio Phys 2020, McDaniel Clin Canc Res 2020, Baschnagel Mol Cancer Ther 2021). We continue to pursue studies to improve treatment outcomes for cancer patients by combining radiation and molecularly targeted agents. For example, we demonstrated that the ATR inhibitor M6620 (VX-970) enhances the effects of radiation in non-small cell lung cancer brain metastasis patient-derived model systems (Molecular Cancer Therapeutics 2021) and that capmatinib, a small molecule inhibitor of the cMET receptor that is overexpressed in lung and other cancers, can radiosensitize cancer with activating alterations (amplification or activating mutations) in cMET (Ramesh Int J Rad Bio Phys 2024). This work catalyzed a new collaboration with the labs of Aaron LeBeau and Reinier Hernandez in which we generated a panel of cMET binding antibodies from camelids. These highly novel antibodies have been tagged with [89]Zr89 and are in development as theranostic agents. We recently submitted a multi-PI R01 to further develop the camelid nanobody for use as an imaging and therapeutic agent in lung cancer. This work is being pursued by a graduate student in the lab, Ms. Rachel Minne, who was recently awarded a spot on the Institute for Clinical and Translational Research TL1 training grant.
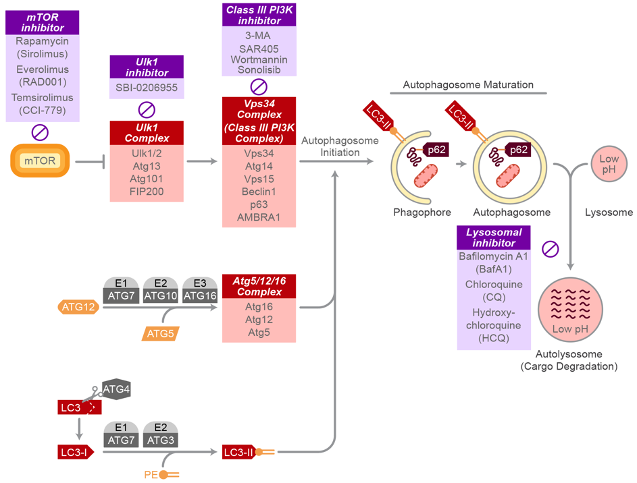
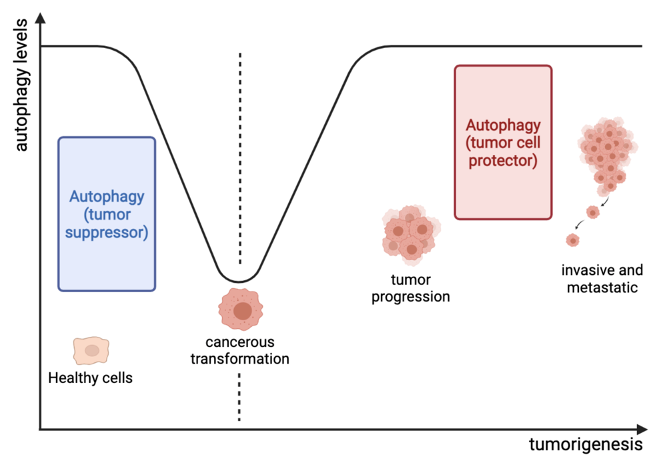
An emerging area of investigation for our group is the interaction between tumor metabolism and the response to radiation. This work is an extension of our American Cancer Society Research Scholar Grant in which we demonstrated that autophagy plays a key role in resistance to cetuximab and radiation. We showed that cetuximab activates autophagy via a LAPTM4B-mediated mechanism. In contrast, radiation-induced autophagy in head and neck cancer proceeds via a Pink1/Parkin-mediated mechanism. We have received pilot funding to study the role of lactate dehydrogenase inhibitors in modulating the immune system and the response to radiation. This work is being led by Dr. Zafer Gurel, a scientist in the lab with expertise in cellular metabolism. We have shown that LDH inhibition conditions the tumor microenvironment to stimulate an anti-tumor immune response and improves the tumor-intrinsic response to radiation therapy.
Cancer cells feature a high degree of plasticity, allowing them to adapt rapidly changing tumor microenvironment through metabolic reprogramming. Cancer cell plasticity, with genetic and epigenetic alterations, promote the diversity of cancer cells and contributes to heterogeneity within the tumor. With this in mind, tumors harbor cells with a wide genetic and metabolic heterogeneity, including cancer stem cells. Thus, a combination of target-specific agents might be required to effectively prevent the development of radio/drug-resistance to eliminate tumor cells entirely. In this manner, we developed a multimodal approach to overcome the potential cellular plasticity and heterogeneity within HNC tumors. Our current studies in this area are aimed at targeting the ROS scavenger system and lactate metabolism in malignancies using rationally developed combination treatments.
Funding
- American Cancer Society Research Scholar Grant
- American Cancer Society Mission Boost Grant
Relevant Publications
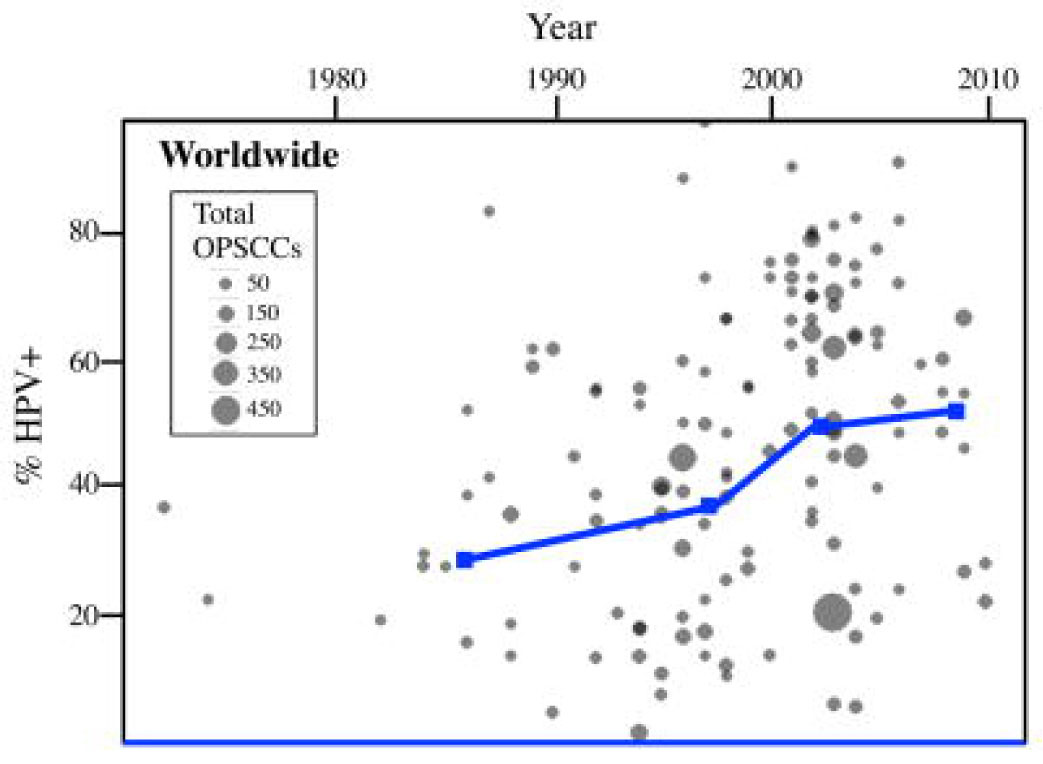
4) Human Papillomavirus Related Head and Neck Cancer
Human papillomavirus (HPV) is a ubiquitious virus that can cause multiple cancers in humans. Our lab has studied HPV-related head and neck cancer and has helped define the molecular basis for the improved outcomes in patients with HPV-related head and neck cancer (in comparison to HPV-unrelated). Multiple clinical trials investigating how we can personalize treatment for patients based on the cause of their cancer are now ongoing. This work has catalyzed clinical studies at UW investigating rapid tumor response assessment in HPV-related HNC.
Relevant Publications
https://pubmed.ncbi.nlm.nih.gov/?term=Kimple+RJ+and+%28HPV+or+human+papillomavirus%29&sort=date

Current Team Members
Principal Investigator
Randall Kimple, MD PhD MBA FASTRO
Dr. Kimple received his MD, PhD and completed residency in radiation oncology at the University of North Carolina and his MBA at Edgewood College. He is a member of the Multidisciplinary Head and Neck Oncology team and leads a group of talented researchers seeking to improve the care of patients with head and neck cancer.
Research Scientists
Kwang P Nickel, PhD
Research Scientist
Dr. Nickel received her PhD. in Nutritional Science at Purdue University. She has been a member of the Kimple Lab since 2012.
She has been involved in various projects including the establishment of Head and Neck PDXs and the investigation of chemo and radiation responses in both in vivo and in vitro HNC models. Her current focus of the study is to characterize the radiobiological effects of different radiation sources such as 137Cs, 60Co, and X-ray. Due to the worldwide radioactive 137Cs irradiator replacement program to the non-radioactive alternative of X-ray irradiation, there is a need to compare these radiation sources for biological effectiveness in both cell culture and animal models since X-ray produces a significantly different energy spectrum compared to 137Cs irradiation.
Zafer Gurel, PhD
Research Scientist
Dr. Gurel received his PhD at the University of Istanbul in Biophysics and has been member of the Kimple Lab since 2019. His focus is on cancer metabolism and in particular how alterations in cancer metabolism influce the tumor immune microenvironment.
Graduate Students
Cristina Paz
Graduate Student, Cancer Biology Program, SciMed GRS, Voice Research Training Program
https://cancerbiology.wisc.edu/
Ms. Paz is pursuing a PhD with her work focused on the use of mesenchymal stromal cells for the treatment of radiation induced xerostomia (dry mouth).
Rachel Minne, MS
Graduate Student, Medical Physics, ICTR TL1 predoctoral training program
https://ictr.wisc.edu/program/tl1-predoctoral-training-program/
Ms. Minne is pursuing a PhD in medical physics. Her work is focused on the development of novel theranostic agents targeting the MET receptor in lung and head and neck cancer.
Liliana Berube
Graduate Student, Medical Physics, SciMed GRS
Ms. Berube is pursuing a PhD in medical physics. Her work is focused on understanding evolution of patient derived model systems and how choices made at the time of model generation influence their ability to be used as faithful avatars of patient response.
Loren Lopez Rivera
Graduate Student, Molecular and Cellular Pharmacology, SciMed GRS
Ms. Lopez Rivera is pursuing a PhD in Molecular and Cellular Pharmacology. Her work is focused on understanding the impact of the immune system on patient derived systems using a multi-omic approach.
Graduate Students
| Name | Undergrad, Predoc, postdoc | Training period,
Awards during training |
Prior Academic Degree(s), Year, Institution | Title of Research Project | Source of Support in Kimple Lab/Position after leaving Kimple Lab |
| Robert Yang | Pre, Univ of Wisconsin | 2010-2012 | BS/BA
2007 University of Wisconsin |
Establishment of primary tumor xenografts from head and neck cancer | Resident
Otolaryngology University of Minnesota Minneapolis, MN |
| Julian Hong | Pre, Univ of Wisconsin | 2010-2014 | BS, 2008
MS, 2009 Stanford University |
Patterns of radiation oncology practice in the longitudinal oncology registry of head and neck carcinoma (LORHAN®) study | Resident
Radiation Oncology Duke University Durham, NC |
| Tyler Fowler | Pre, Univ of Wisconsin
Co-mentor with Bryan Bednarz |
2011 – 2015
2011 – Advanced opportunity fellowship graduate research scholar 2011 – Biological Science Scholar 2013 – 4th Annual Standard Imaging Travel Award. American Association of Physicists in Medicine Annual Meeting 2013 – igus Young Engineer’s Support Program Award |
BS, 2011 Southern Oregon University | Development and biovalidation of a high-throughput microirradiator for the study of autophagy in head and neck cancer | Resident in Medical Physics
Stanford University Palo Alto, CA |
| Andy Stein | Pre, Univ of Wisconsin | 2013-2015
Graduation with Research Honors 2013-2014 Shapiro Fellowship |
BS, 2009 Washington University | Molecular markers of response in head and neck cancer xenografts | Resident
Otolaryngology Case Western Reserve University School of Medicine Cleveland, OH |
| Stephanie Rice | Pre, Univ of Wisconsin | 2013-2014
Graduation with Research Honors |
BS, 2007
UW Lacrosse |
Skin cancers of the head and neck. | Resident
Radiation Oncology University of Maryland Baltimore, MD |
| Evan Liang | Pre, Univ of Wisconsin | 2014-2017
2014 Shapiro Summer Research Fellow |
BS, 2012
Harvard University |
Outcomes in Merkel Cell Carcinoma | Resident
Radiation Oncology Wayne State University |
| Leonard Che Fru | Pre, Univ of Wisconsin, co-mentor with Larry DeWerd | 2013-2019 | BS 2008 Minnesota State Univ
MS, 2012 Minnesota State Univ |
Measurement of hemoglobin oxygen saturation in tissue with an optical device | Resident in Medical Physics
University of Wisconsin Madison, WI |
| Yong-Syu (Aaron) Lee | Pre, Univ of Wisconsin | 2017-2024 | BS, 2008 China Medical University, Taichung Taiwan
MS, 2010 National Yang-Ming University, Taipei Taiwan |
Therapy induced autophagy in head and neck cancer | American Cancer Society grant (Kimple PI) |
Postdoctoral Fellows
| Name | Undergrad, Predoc, postdoc | Training period,
Awards during training |
Prior Academic Degree(s), Year, Institution | Title of Research Project | Source of Support in Kimple Lab/Position after leaving Kimple Lab |
| HaoShun Huang | Postdoc, Univ of Wisconsin | 2012-2013 | PhD, 2012, Univ of Wisconsin | Genomic and therapeutic characterization of primary tumor xenografts from head and neck cancer | Medical Science Liaison, Sanofi |
| Adam Swick | Postdoc, Univ of Wisconsin | 2013 – 2017
2015 AACR Scholar in Training Travel Award 2015 PhRMA Foundation Translational Science Postdoctoral Fellow |
PhD, 2013, Univ of Wisconsin | Molecular targeting of head and neck cancer to overcome therapeutic resistance | Project Manager, Catalent |
| John Floberg | Postdoc, Univ of Wisconsin | 2013-2014 | PhD, 2012, Univ of Wisconsin | Correlation of FDG-PET with response in oropharyngeal cancers. | Resident in Radiation Oncology, Washington University in St Louis |
| Anirban Chatterjee | Postdoc, Univ of Wisconsin | 2015-2017 | PhD, 2014, Univ of Calcutta | Head and Neck Cancer Stem Cells | Assistant Professor
Bolpur College, University of Burdwan, Bolpur, Birbhum, West Bengal, India |
| Jaimee Eckers | Postdoc, Univ of Wisconsin | 2016-2018 | PhD, 2013, Univ of Iowa | Autophagy in head and neck cancer | Assistant Professor
Nevada State College Henderson, NV |
Medical Residents/Fellows
| Name | Training period,
Awards during training |
Prior Academic Degree(s), Year, Institution | Title of Research Project | Position after leaving Kimple Lab or Current Position |
| Stephen Rosenberg | 2014-18 | MD, 2013, Rutgers | Readability of online patient information | Assistant Professor
H. Lee Moffit Cancer Center Tampa, FL |
| H. Cindy Ko | 2015-2019 | MD, 2014, New York University | Mindfulness meditation to reduce physician burnout | Kaiser Permanente of Southern California, Los Angeles, CA |
| Grace Blitzer | 2018-2023
2020 RSNA Research Resident Award 2021 ASCO Young Investigator Award |
MD, 2017, Medical College of Wisconsin | Treatments for radiation induced xerostomia | Resident, Radiation Oncology, University of Wisconsin |
| Charles Gast | 2020 | MD PhD, Oregon Health & Science University | Treatments for radiation induced xerostomia | Resident, Otolaryngology, University of Wisconsin |
Others
| Name | Title | Period | Prior Academic Degree(s), Year, Institution | Title of Research Project | Current Position/Source of Support |
| Lindsey Abel | Research Specialist | 2017-2021 | University of Missouri – Columbia | Multiple | Data Management Coordinator, Children’s Healthcare of Atlanta, Atlanta GA |
| Holly Edwards | Undergrad, Univ of North Carolina | 2006-2007 | DNA damage modulation by HER4 in breast cancer | Pediatric resident at Palmetto Health Children’s Hospital, Columbia, SC | |
| Jill Zartman | Undergrad, Univ of North Carolina | 2008-2010 | Nutritional needs assessment in radiation oncology clinics | Service Member at FoodCorps | |
| Christopher Harris | Predoc, Univ of North Carolina | 2008-2010 | BS
2007 East Carolina University |
Validation of pazopanib as a lung cancer radiosensitizer | Psychiatry Resident US Navy |
| Timothy Baerg | Undergrad, Univ of Wisconsin | 2011-2012
· 2011 UW Undergraduate Research Scholar |
Assessment of apoptosis in head and neck cancer after radiation | Medical Student (MD/MBA)
University of Michigan Ann Arbor, MI |
|
| Molly Smith | Undergrad, Univ of Wisconsin | 2010 – 2013
· 2012 AACR Undergraduate Poster Competition 3rd Place Award |
Cell cycle differences in HPV-positive and HPV-negative head and neck cancer | Graduate student
Cancer Biology University of Cincinnati Cincinnati, OH |
|
| Grace Blitzer | Undergrad, Univ of Wisconsin | 2010-2013
· 2011 UW Hilldale Undergraduate Research Fellow · 2012-13 AACR Thomas J. Bardos Science Education Award for Undergraduate Students · 2012 Senior Honors Thesis Grant · 2013 University Book Store Academic Excellence Award |
Radiation and chemotherapy response profiles of HPV-positive head and neck cancer | Medical student
Medical College of Wisconsin Milwaukee, WI |
|
| Kai Ludwig | Undergrad, Univ of Wisconsin | 2012-2013 | Autophagy in head and neck cancer | Graduate student
Department of Medical Physics University of Wisconsin Madison, WI |
|
| Alexandra Torres | Pre, Univ of Wisconsin | 2012 – 2013
2012 – Advanced opportunity fellowship graduate research scholar 2013 – Virology Training Grant |
BS, 2011 Harvard University | HPV regulation of EGFR expression | Graduate Student
Cancer Biology Training Program Department of Oncology University of Wisconsin Madison, WI UW Advanced opportunity fellowship, Virology Training Grant |
| Ebony Carson | Undergrad, Univ of Wisconsin | 2012 – 2016
2012 UW Undergraduate Research Scholar |
Effect of p53 reactivation in HPV-positive head and neck cancer. | Clinical Research Coordinator
Medical College of Wisconsin Milwaukee, WI |
|
| Divya Bhat | Undergrad, Univ of Wisconsin | 2012 – 2016
2012 UW Undergraduate Research Scholar |
Generation of HPV-16 E6 mutant cell lines. | Graduate student, University of Iowa, Iowa City, IA | |
| Dana Gunderson | Undergrad, Univ of Wisconsin | 2013-2015 | Characterization of radiation response in a new HPV-positive HNC cell line. | Dental School,
Marquette University Milwaukee, WI |
|
| Ali Bailey | Undergrad, Univ of Wisconsin | 2013 – 2015
2015 AACR Undergraduate Poster Competition Honorable Mention |
Autophagy in Head and Neck cancer | Medical Student
Loyola University Chicago, IL |
|
| Michael Fisher | Undergrad, Univ of Wisconsin | 2014 – 2017
2016 UW Hilldale Undergraduate Research Fellow |
Effects of HPV oncoprotein mutation on head and neck cancer radiation sensitivity | Medical Student
University of Chicago Chicago, IL |
|
| Prashanth Prabakaran | Predoc, Univ of Wisconsin | 2015-2016 | BS, University of Wisconsin | Molecular targeting of Adenoid Cystic Carcinoma | Medical Student
University of Wisconsin Madison, WI |
| Aastha Pandey | Undergrad, Univ of Wisconsin | Summer 2015 – high school
2015 Science Research Internship Program 2016 – 2020 |
Radiosensitization of bladder cancer | Undergraduate
University of Wisconsin Madison, WI |
|
| Justin Skiba | Undergrad, Univ of Wisconsin | 2015 – 2019 | Autophagy in head and neck cancer | Medical Student
University of Pittsburgh Pittsburgh, PA |
|
| Margot Miller | Undergrad, Univ of Wisconsin | 2015 – 2019
2018 UW Hilldale Undergraduate Research Fellow 2019 David Boren Scholar 2019 Lauguage Flagship Scholar |
Dual targeting of EGFR and MTORC in HNC | Russian Flagship Program, Almaty, Kazakhstan | |
| Amal Javaid | Predoc, Univ of Wisconsin | 2016 – 2018 | BS, University of Wisconsin | Radiosentiziation of adenoid cystic carcinoma | Medical Student
University of Pittsburgh Pittsburgh, PA |
| Austin Maas | Predoc, Univ of Wisconsin | 2016 – 2018 | BS, University of Michigan | Head and neck cancer initiating cells | Medical Student
University of Michigan Ann Arbor, MI |
| Gopika Senthikumar | Undergrad, Univ of Wisconsin | 2016 – 2019
2018 UW Hilldale Undergraduate Research Fellow |
Autophagy in head and neck cancer | MD PhD Student
Medical College of Wisconsin Milwaukee, WI |
|
| Amber Bo | Undergrad, Univ of Wisconsin | 2017 – 2019 | AXL in head and neck cancer | Medical Student
Medical College of Wisconsin Milwaukee, WI |
|
| Ashley Kromke | Undergrad, Univ of Wisconsin | 2018 – 2021 | FGFR in lung cancer | Undergraduate
University of Wisconsin Madison, WI |
|
| Samantha Bradley | Undergrad, Univ of Wisconsin | 2019 – pres
2020 UW Sophomore Research Award 2021 UW Hilldale Undergraduate Research Fellowship |
Autophagy in head and neck cancer | Undergraduate
University of Wisconsin Madison, WI |
|
| Haley VanBeek | Undergrad, Univ of Wisconsin | 2019 – 2021 | ATM inhibition in lung cancer | Medical Student
Medical College of Wisconsin Milwaukee, WI |
|
| Lexi Luo | Undergrad, Univ of Wisconsin | 2018-2022
2021 Goldwater Fellowship 2021 Biochemistry Research Award 2021 UW Hilldale Undergraduate Research Fellowship 2021 Astronaut Foundation Scholar |
Metabolic alterations to increase sensitivity to radiation in head and neck cancer | Undergraduate
University of Wisconsin Madison, WI |
|
| Mitchell Boettner | Undergrad, Univ of Wisconsin | 2019-2020 | Autophagy in head and neck cancer | Undergraduate
University of Wisconsin Madison, WI |
|
| Annie Glassey | Undergrad, Univ of Wisconsin | 2020-2022
2021 UW Hilldale Undergraduate Research Fellowship |
Cell therapy to prevent and treat radiation induced xerostomia | Undergraduate
University of Wisconsin Madison, WI |
|
| Shrey Ramesh | Undergrad, Univ of Wisconsin | 2021-2024
2021 UW Sophomore Research Award |
RBE determination of electronic brachytherapy sources | Undergraduate
University of Wisconsin Madison, WI |
|
| Michael Luy | Undergrad, Univ of Wisconsin | 2019-2022 – worked as a research intern untill May of 2023 | Metabolic alterations to increase sensitivity to radiation in head and neck cancer | Undergraduate University of Wisconsin
Madison, WI |
Album
Locations
University Hospitals and Clinics
Clinical trials
I play an active role in the UW Head and Neck Cancer Disease Oriented Team which oversees and prioritizes head and neck cancer focused clinical research activities at the University of Wisconsin. In addition, I am an active participant and former co-chair of the Big Ten Cancer Research Consortium Head and Neck Cancer Working Group. This group focuses on early phase multi-institutional clinical trials for patients with head and neck cancers.
Through clinical trials I hope to bring promising treatments to patients and integrate advanced imaging, novel molecular tests, and molecular markers to match each patient to the right treatment for their individual cancer.
We have recently completed two pilot studies in patients with head and neck cancer that have paved the way to an ongoing phase 1 study investigating the safety and tolerability of bone marrow derived mesenchymal stromal cells for treatment of radiation-induced salivary dysfunction.
I also serve as translational science co-chair on the recently approved NRG HN012 study.
Relevant Publications
https://pubmed.ncbi.nlm.nih.gov/?term=Kimple+RJ+and+%28IRB+OR+Review%29&sort=date

In addition to my clinical and research roles, I teach undergraduate, graduate and medical students as well as residents and postdoctoral fellows. I try to give these students a sense of the breadth of opportunities in medicine and challenge them to be the best doctors and scientists they can be. I encourage my students to come to the clinic with me so that they can see cancer from our patient’s perspective.
I serve as Faculty Director of the Graduate Program in Clinical Investigation https://ictr.wisc.edu/graduate-program-in-clinical-investigation/ which is an applied degree program in which trainees focus on the creation of novel methodologies and tools for translational science within the context of a specific biomedical discipline. The GPCI is supported by the UW Institute for Clinical and Translational Research https://ictr.wisc.edu/.
-
Still thirsting for a fix Translational cancer research
Blitzer GC, Galipeau J, McCoy SS, Kimple RJ
2025 Apr 30;14(4):2175-2177. doi: 10.21037/tcr-2025-150. Epub 2025 Apr 15.
-
Clinicopathologic and Molecular Characterization of SMARCB1-Deificient Sinonasal Carcinomas -A Systematic Study from a Single Institution Cohort Head and neck pathology
Li Q, Abi-Saab T, Prilutskiy A, Horner V, Frater-Rubsam L, Peng Y, Huang W, Kimple RJ, Harari PM, Lloyd RV, Hu R
2025 May 14;19(1):60. doi: 10.1007/s12105-025-01788-w.
-
More
BACKGROUND: SMARCB1-deficient and SMARCA4-deficient sinonasal carcinomas are rare, with only a few systematic studies available in the literature. Secondary EWSR1 gene abnormalities have been reported in SMARCB1-deficient tumors. This study aimed to systematically investigate SWI/SNF complex-deficient sinonasal carcinomas in a single-institution cohort, perform clinicopathologic characterization, and explore the underlying molecular mechanisms.
METHOD: Immunohistochemistry (IHC) of INI1 and BRG1 was performed on tissue microarrays containing tumor tissue from 149 consecutive sinonasal carcinomas. Single nucleotide polymorphism (SNP) array and EWSR1 gene fluorescence in situ hybridization (FISH) analyses were conducted on SMARCB1-deficient sinonasal carcinomas. Clinicopathologic characterization was studied.
RESULT: Of the 149 sinonasal carcinomas, 7 (4.7%) showed SMARCB1 loss, while none demonstrated SMARCA4 loss. All patients were male and presented with advanced-stage tumors. Four SMARCB1-deficient sinonasal carcinomas exhibited basaloid morphology, two displayed eosinophilic tumor morphology, and one had mixed morphology. Homozygous and heterozygous SMARCB1 deletions were identified in 4/6 and 2/6 cases respectively. Heterozygous loss involving genes neighboring SMARCB1 gene, including EWSR1, was observed in four cases. One tumor showed a heterozygous loss of the entire chromosome 22q. EWSR1 FISH assay revealed concordant heterozygous EWSR1 loss in these five cases.
CONCLUSION: SMARCB1-deficient carcinomas account for 4.7% of sinonasal carcinomas in this single-institution cohort, while SMARCA4-deficient tumors are even rarer, with none identified. SMARCB1-deficient sinonasal carcinomas exhibit a broad spectrum of morphologic and immunohistochemical features. These carcinomas show complex genetic alterations, with homozygous SMARCB1 deletions present in the majority of cases.
PMID:40366517 | PMC:PMC12078905 | DOI:10.1007/s12105-025-01788-w
View details for PubMedID 40366517
-
More
-
National Institutes of Health Funding to Support Radiation Oncology Research: A Comparative Trend Analysis Over a Decade, 2011-2021 Advances in radiation oncology
Razavi A, Rooney MK, Fuller CD, Yu JB, Pfister NT, Thomas CR, Buatti JM, Kamran SC, McGee HM, Yeboa DN, Kiess AP, Baschnagel AM, Kimple RJ
2025 Apr 22;10(6):101767. doi: 10.1016/j.adro.2025.101767. eCollection 2025 Jun.
-
More
PURPOSE: Funding to support radiation oncology discovery and research is essential for advancement in therapeutic strategies to improve outcomes for patients with cancer. We aimed to comprehensively characterize trends in National Institutes of Health (NIH) funding that supports radiation oncology research over time to identify trends, successes, and areas for improvement.
METHODS AND MATERIALS: We queried the NIH Research Portfolio Online Reporting Tools Expenditures and Results database to identify all awarded grants to support radiation oncology research conducted by principal investigators at academic centers, using 3 individual years as representative samples (2011, 2016, and 2021). Abstracts and keywords for resulting grants were manually searched to identify resulting awards topically related to the field of radiation oncology; principal investigators departmental affiliation was also used as a supplemental method serving as a sensitivity analysis to define radiation oncology-related research. Descriptive statistics were used to describe patterns in funding. χ2 testing was used to assess differences in proportions of categorical variables.
RESULTS: Less than 0.5% of the total NIH budget and < 2% of the total National Cancer Institute budget supported radiation oncology research during the representative study years. There were no significant changes in this allocation pattern over time. A small cohort of institutions held a relatively large proportion of NIH-supported radiation oncology grant funding. Individuals holding PhDs alone received the majority of funding (62%), whereas those with dual-degrees (MD/PhD) held 21% of funding, and those with MD alone were awarded 17% of funding. There was a trend toward an increased proportion of grants awarded to MD/PhDs over time (24% vs 15% in 2021 and 2011, respectively, P = .075).
CONCLUSIONS: Despite radiation therapy's essential role in multidisciplinary cancer care, NIH, and National Cancer Institute funding to support radiation oncology research has remained disproportionally low over the last decade. These data may be useful to inform future policy aimed at promoting research advancement in radiation oncology both at the micro (individual) as well as macro (institutional and national) level.
PMID:40330712 | PMC:PMC12051116 | DOI:10.1016/j.adro.2025.101767
View details for PubMedID 40330712
-
More
-
Subclonal response heterogeneity to define cancer organoid therapeutic sensitivity Scientific reports
Kratz JD, Rehman S, Johnson KA, Gillette AA, Sunil A, Favreau PF, Pasch CA, Miller D, Zarling LC, Yeung AH, Clipson L, Anderson SJ, Steimle AK, Sprackling CM, Lemmon KK, Abbott DE, Burkard ME, Bassetti MF, Eickhoff JC, Foley EF, Heise CP, Kimple RJ, Lawson EH, LoConte NK, Lubner SJ, Mulkerin DL, Matkowskyj KA, Sanger CB, Uboha NV, Mcilwain SJ, Ong IM, Carchman EH, Skala MC, Deming DA
2025 Apr 9;15(1):12072. doi: 10.1038/s41598-025-96204-2.
-
More
Tumor heterogeneity is predicted to confer inferior clinical outcomes with precision-based strategies, however, modeling heterogeneity in a manner that still represents the tumor of origin remains a formidable challenge. Sequencing technologies are limited in their ability to identify rare subclonal populations and predict response to treatments for patients. Patient-derived organotypic cultures have significantly improved the modeling of cancer biology by faithfully representing the molecular features of primary malignant tissues. Patient-derived cancer organoid (PCO) cultures contain subclonal populations with the potential to recapitulate heterogeneity, although treatment response assessments commonly ignore diversity in the molecular profile or treatment response. Here, we demonstrate the advantage of evaluating individual PCO heterogeneity to enhance the sensitivity of these assays for predicting clinical response. Additionally, organoid subcultures identify subclonal populations with altered treatment response. Finally, dose escalation studies of PCOs to targeted anti-EGFR therapy are utilized which reveal divergent pathway expression when compared to pretreatment cultures. Overall, these studies demonstrate the importance of population-based organoid response assessments, the use of PCOs to identify molecular heterogeneity not observed with bulk tumor sequencing, and PCO heterogeneity for understanding therapeutic resistance mechanisms.
PMID:40200028 | PMC:PMC11978853 | DOI:10.1038/s41598-025-96204-2
View details for PubMedID 40200028
-
More
-
PRAME Epitopes are T-Cell Immunovulnerabilities in <em>BRD4::NUTM1</em> Initiated NUT Carcinoma bioRxiv : the preprint server for biology
Jensen JL, Peterson SK, Yu S, Kinjo T, Price BA, Sambade M, Vesko S, DeBetta JD, Geyer JK, Nickel KP, Kimple RJ, Kotecha RS, Davis IJ, Wang JR, French CA, Kuhlman B, Rubinsteyn A, Weiss J, Vincent BG
2025 Mar 12:2025.03.07.642090. doi: 10.1101/2025.03.07.642090.
-
More
NUT carcinoma ("NC") is a rare but highly lethal solid tumor without an effective standard of care. NC is caused by bromodomain-containing NUTM1 fusion genes, most commonly BRD4::NUTM1 . BRD4::NUTM1 recruits p300 to acetylate H3K27 forming "megadomains" with the overexpression of encapsulated oncogenes, most notably MYC . Akin to MYC , we hypothesized that transcriptional dysregulation caused by BRD4::NUTM1 would lead to the generation of cancer specific antigens that could be therapeutically actionable. Integrating genomics, immunopeptidomics, and computational biology approaches, we identified PRAME as the predominantly transcribed and HLA Class I-presented cancer/testis antigen in NC. Further, we show that a PRAME epitope-specific T-cell receptor ("TCR") x CD3 activator bispecific molecule modeled after brenetafusp has potent T-cell mediated activity against PRAME+ NC. Our results show that PRAME is often highly expressed in NC due to BRD4::NUTM1, and that BRD4::NUTM1 induced PRAME antigens are promising TCR targets for forthcoming clinical trials in NC.
STATEMENT OF SIGNIFICANCE: NC is one of the most aggressive solid tumors to afflict humans and is refractory to chemotherapy, T-cell checkpoint blockade, and targeted therapies. We show PRAME epitopes are promising targets for TCR-based therapeutics like brenetafusp in NC, adding to growing momentum for addressing challenging fusion malignancies with TCR therapeutics.
PMID:40161761 | PMC:PMC11952323 | DOI:10.1101/2025.03.07.642090
View details for PubMedID 40161761
-
More
-
Radiation-Therapy Related Salivary Dysfunction Seminars in radiation oncology
Blitzer GC, Paz C, McCoy SS, Kimple RJ
2025 Apr;35(2):278-284. doi: 10.1016/j.semradonc.2025.02.006.
-
More
Radiation-induced xerostomia (RIX) is a common and debilitating side effect of head and neck cancer radiotherapy, significantly impacting patients' quality of life. This review comprehensively summarizes the current understanding of RIX, encompassing its clinical quantification, underlying pathophysiology, and established and emerging treatment modalities. We explore various objective and subjective measures used to quantify salivary flow and assess the severity of xerostomia in clinical settings. The pathophysiological mechanisms leading to RIX are elucidated, including radiation damage to salivary glands, alterations in saliva composition, and the role of inflammatory processes. Current treatment strategies, such as saliva substitutes and stimulants, are discussed alongside their limitations. Furthermore, we delve into novel investigational approaches, including gene therapy, stem cell transplantation, and pharmacologic interventions, offering promising avenues for future RIX management. This review provides clinicians and researchers with a comprehensive overview of RIX, highlighting the need for continued research to develop more effective preventative and therapeutic strategies to alleviate this burdensome condition.
PMID:40090753 | PMC:PMC11911547 | DOI:10.1016/j.semradonc.2025.02.006
View details for PubMedID 40090753
-
More
-
Acrylamide-based Saliva-gels as a Potential Xerostomia Treatment ACS applied polymer materials
Debnath S, Woeppel AB, Paz C, Rogus-Pulia N, Kimple RJ, Malandraki GA, Boudouris BW
2023 Oct 13;5(10):7698-7704. doi: 10.1021/acsapm.3c00438. Epub 2023 Sep 18.
-
More
Xerostomia often introduces impactful deficits in swallowing, oral communication, and oral hygiene. Current treatments only temporarily reduce symptoms without offering long-term benefits. While efforts to develop implantable salivary glands are ongoing, a cost-effective, easy-to-use, and non-invasive solution has yet to present itself. Here, we utilize biocompatible, acrylamide-based saliva-gels to control artificial saliva release and mimic natural saliva release properties. Specifically, we synthesized saliva-gels and tailored their mechanical properties (e.g., their tensile strength) by adjusting the loading of saliva in the polymer-based saliva-gels. Importantly, all saliva-gels were capable of swelling to ~ 650% of their initial volume over short time periods (3 h). Moreover, the optimized saliva-gel formulation released about 80% of the artificial saliva stored in its network within 24 h at a temperature of 37 °C (i.e., human body temperature). Additionally, the saliva released by the saliva-gels was chemically identical to that originally absorbed. This controlled release profile offers a proof-of-concept demonstration for the use of these materials in future xerostomia treatment applications.
PMID:40061240 | PMC:PMC11887651 | DOI:10.1021/acsapm.3c00438
View details for PubMedID 40061240
-
More
-
Exercise May Improve Completion of Standard and Emerging Cancer Treatments Exercise and sport sciences reviews
Catalá-Vilaplana I, Cao SE, Zadravec K, LeVasseur N, Kimple RJ, Lim AJ, Courneya KS, Campbell KL
2025 Jul 1;53(3):110-124. doi: 10.1249/JES.0000000000000360. Epub 2025 Feb 18.
-
More
Receipt of the entire course of intended anticancer treatment is critical to maximize treatment efficacy, reduce risk of disease recurrence, and improve survival. Engaging in an exercise program during cancer treatment has the potential to improve treatment completion, but standardization in terminology for reporting on cancer treatment completion is needed, especially as types of cancer treatments continue to evolve.
PMID:39982316 | PMC:PMC12180392 | DOI:10.1249/JES.0000000000000360
View details for PubMedID 39982316
-
More
-
Loss of MK2 Enhances Radiation-Mediated Apoptosis in Bladder Cancer World journal of oncology
Morgan D, Berggren KL, Millington G, Smith H, Spiess C, Hixon M, Woolbright BL, Taylor JA, Kimple RJ, Chen R, Shen X, Gan GN
2024 Dec;15(6):871-881. doi: 10.14740/wjon1945. Epub 2024 Dec 11.
-
More
BACKGROUND: Bladder cancer patients unable to receive cystectomy or who choose to pursue organ-sparing approach are managed with definitive (chemo)radiotherapy. However, this standard of care has not evolved in decades and disease recurrence and survival outcomes remain poor. Identifying novel therapies to combine with radiotherapy (RT) is therefore paramount to improve overall patient outcomes and survival. One approach is to find cellular mechanisms that can be targeted to increase the radiosensitivity of bladder cancer. The stress-activated kinase directly downstream from p38 mitogen-activated protein kinase (MAPK), mitogen-activated protein kinase activated protein kinase 2 (MAPKAPK2 or MK2), has been shown to enhance cancer-mediated inflammation, mesenchymal gene expression, and in vivo tumor growth. Here we examined the impact that MK2 knockdown (KD) has on bladder cancer cell radiosensitivity.
METHODS: We utilized short hairpin RNA (shRNA) KD of MK2 using lentiviral transfection in the bladder cancer cell lines, T24 and HTB9. We compared the growth of KD cells to wild type using colony formation assays, proliferation assays and cell counts to determine differences in cell growth. Apoptosis was examined by annexin-based flow cytometry and western blots. Flow cytometry was also used for cell cycle analysis.
RESULTS: KD clones showed a greater than 90% inhibition of MK2 expression as determined by western blot. Clonogenic assays exhibited an increase in radiosensitivity among the MK2 KD bladder cancer cells. These data were supported with proliferation assays that displayed a greater reduction in cell number following RT in MK2 KD bladder cancer cells. Annexin V binding in bladder cancer cells suggested increased apoptosis in MK2 KD cells. This was confirmed by comparing the amount of cleaved caspase products for the caspases 3 and 8 to scrambled control (SCR), and the release of cytochrome C into the cytosol. Both cell types showed disruptions in the cell cycle but at different points in the cycle.
CONCLUSION: These results show that MK2 controls irradiation-induced apoptosis in bladder cancer cells.
PMID:39697425 | PMC:PMC11650613 | DOI:10.14740/wjon1945
View details for PubMedID 39697425
-
More
-
Safety and toxicity of Iopofosine I 131 (CLR 131) with external beam radiation therapy in recurrent or metastatic head and neck cancer: results of a phase 1 single-centre, open-label, single-arm, dose escalation and dose expansion study EBioMedicine
Bruce JY, Burr A, Kimple RJ, Adam DP, Yu M, Piaskowski SM, Glazer TA, Hill P, Hartig GK, McCulloch TM, Wieland AM, Trask D, Oliver K, Longcor J, Rogus-Pulia N, Cho SY, Bednarz B, Harari PM
2025 Jan;111:105496. doi: 10.1016/j.ebiom.2024.105496. Epub 2024 Dec 12.
-
More
BACKGROUND: Re-irradiation of recurrent head and neck cancer (HNC) is often limited by tumour adherence to critical structures and/or radiation tolerance of critical normal tissues. Iopofosine I 131 (CLR 131) is a targeted small molecular phospholipid ether (PLE) drug conjugate that delivers iodine-131 selectively to tumour cells. We conducted a phase 1, single-centre, open-label study to determine whether CLR 131 given with reduced dose of external beam radiation therapy (EBRT) would be tolerable and feasible.
METHODS: All participants received previous curative intent treatment with radiotherapy as primary or adjuvant treatment. Eligible participants demonstrated uptake of CLR 131 as indicated via single photon emission CT/CT (SPECT/CT) imaging following CLR 131 test dose. Participants received two therapeutic doses of CLR 131 (days 1 and 8) with SPECT/CT imaging performed to quantitate the biodistribution of CLR 131. Participants subsequently received EBRT to achieve the designated radiation dose (60-70 Gy). The primary endpoint was safety. This trial was registered with ClinicalTrials.gov, NCT04105543, and enrolment and follow-up are complete.
FINDINGS: Twelve participants completed treatment with CLR 131 and EBRT. Eight participants experienced grade 4 non-DLT haematologic toxicities (2 anaemia, 8 leukopenia, 5 thrombocytopenia) at least probably attributed to CLR 131, consistent with the expected toxicity profile. Haematologic toxicities occurred during weeks 6-8 from the first dose of CLR 131 and resolved within three weeks without sequelae. There were no treatment-related grade 3-4 non-haematologic toxicities.
INTERPRETATION: CLR 131 in combination with EBRT did not confer any safety concerns, and was tolerable in participants with recurrent/metastatic HNC. Myelosuppression was consistent with the known toxicity profile of CLR 131.
FUNDING: National Institutes of HealthP50 DE026787, National Cancer InstituteP30 CA014520, National Institutes of Health1UL1TR002373, Cellectar, NCT04105543.
PMID:39671752 | PMC:PMC11700259 | DOI:10.1016/j.ebiom.2024.105496
View details for PubMedID 39671752
-
More
-
Evaluation of a Novel MET-Targeting Camelid-Derived Antibody in Head and Neck Cancer Molecular pharmaceutics
Minne RL, Luo NY, Mork CM, Wopat MR, Esbona K, Javeri S, Nickel KP, Hernandez R, LeBeau AM, Kimple RJ, Baschnagel AM
2024 Dec 2;21(12):6376-6384. doi: 10.1021/acs.molpharmaceut.4c00938. Epub 2024 Nov 8.
-
More
In head and neck squamous cell carcinoma (HNSCC), the mesenchymal epithelial transition (MET) receptor drives cancer growth, proliferation, and metastasis. MET is known to be overexpressed in HNSCC and, therefore, is an appealing therapeutic target. In this study, we evaluated MET expression in patients with HNSCC and investigated the potential imaging application of a novel MET-binding single-domain camelid antibody using positron emission tomography/computed tomography (PET/CT) in a preclinical MET-expressing HNSCC model. Multiplex immunostaining for MET protein performed on a tissue microarray from 203 patients with HNSCC found 86% of patients to have MET expression, with 14% having high expression and 53% having low MET expression. Using The Cancer Genome Atlas (TCGA) database, high MET RNA expression was associated with worse progression-free survival and overall survival in patients with HPV-negative HSNCC. Utilizing flow cytometry and immunofluorescence, our novel camelid antibody fused to a human IgG Fc chain (1E7-Fc) showed high binding affinity and specificity to high MET-expressing Detroit 562 cells but not to low MET-expressing HNSCC cells. The efficacy and biodistribution of [89Zr]Zr-1E7-Fc as a PET imaging agent was then investigated in a MET-expressing head and neck xenograft model. [89Zr]Zr-1E7-Fc rapidly localized and showed high tumor uptake in Detroit 562 xenografts (8.4% ID/g at 72 h postinjection), with rapid clearance from the circulatory system (2.7 tumor-to-blood radioactivity ratio at 72 h postinjection). Our preclinical data suggest that the camelid antibody 1E7-Fc could be a potential theranostic agent for HNSCC. Further investigations are warranted to confirm these findings in patients and to evaluate 1E7-Fc as an imaging agent and platform to deliver radionuclide or drug therapy to MET-driven cancers.
PMID:39513517 | PMC:PMC11987585 | DOI:10.1021/acs.molpharmaceut.4c00938
View details for PubMedID 39513517
-
More
-
From Bench to Bedside: A Team's Approach to Multidisciplinary Strategies to Combat Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma Journal of clinical medicine
Crossman BE, Harmon RL, Kostecki KL, McDaniel NK, Iida M, Corday LW, Glitchev CE, Crow MT, Harris MA, Lin CY, Adams JM, Longhurst CA, Nickel KP, Ong IM, Alexandridis RA, Yu M, Yang DT, Hu R, Morris ZS, Hartig GK, Glazer TA, Ramisetty S, Kulkarni P, Salgia R, Kimple RJ, Bruce JY, Harari PM, Wheeler DL
2024 Oct 10;13(20):6036. doi: 10.3390/jcm13206036.
-
More
Head and neck squamous cell carcinoma (HNSCC) is diagnosed in more than 71,000 patients each year in the United States, with nearly 16,000 associated deaths. One significant hurdle in the treatment of HNSCC is acquired and intrinsic resistance to existing therapeutic agents. Over the past several decades, the University of Wisconsin has formed a multidisciplinary team to move basic scientific discovery along the translational spectrum to impact the lives of HNSCC patients. In this review, we outline key discoveries made throughout the years at the University of Wisconsin to deepen our understanding of therapeutic resistance in HNSCC and how a strong, interdisciplinary team can make significant advances toward improving the lives of these patients by combatting resistance to established therapeutic modalities. We are profoundly grateful to the many scientific teams worldwide whose groundbreaking discoveries, alongside evolving clinical paradigms in head and neck oncology, have been instrumental in making our work possible.
PMID:39457986 | PMC:PMC11508784 | DOI:10.3390/jcm13206036
View details for PubMedID 39457986
-
More
-
Uncommon and Challenging Phenotypes of High-Risk Human Papillomavirus-Associated Head and Neck Carcinomas Revealed by High-Throughput Studies Head and neck pathology
Tannenbaum AP, Lozar T, Lu C, Schumacher M, Golfinos A, Dinh HQ, Taylor N, Kimple RJ, Yang D, Harari PM, Lambert PF, Lloyd RV, Hu R
2024 Oct 22;18(1):112. doi: 10.1007/s12105-024-01707-5.
-
More
BACKGROUND: HPV- associated squamous cell carcinoma (SCC) is uncommon in non-oropharynx sites and not well characterized. This study aims to investigate uncommon phenotypes of HPV-associated head and neck carcinoma, the prevalence and morphologic spectrum of HPV-associated SCC in the oral cavity, larynx and hypopharynx.
METHOD: P16 immunostaining and HPV E6/7 in situ hybridization (ISH) were performed on tissue microarrays comprised of SCCs from different anatomic sites: oropharynx (n = 270), hypopharynx (n = 52), oral cavity (n = 95) and larynx (n = 123). Tumors were classified as HPV-associated based on a positive E6/7 ISH testing. RNA sequencing was performed on several selected cases.
RESULT: 66% oropharynx SCCs (OPSCCs) were HPV-associated; all were p16/HPV testing concordant except one which was p16 negative. The p16-/HPV + OPSCC resembled similar gene expression signature with p16+/HPV + OPSCCs by transcriptome analysis. 6/95 (6%) oral cavity SCCs were HPV-associated, all from male patients and 5/6 (83%) arose from the floor of mouth. Morphologically, 3/6 (50%) showed keratinizing SCC and 5/6 (83%) demonstrated HPV-associated squamous dysplasia in adjacent mucosa. 1/123 (less than 1%) larynx SCCs and 0/52 hypopharynx SCCs were HPV-associated.
CONCLUSION: Although uncommon, p16 negative HPV-associated OPSCC can occur, emphasizing the importance of judicious HPV testing. The morphology of HPV-associated oral cavity SCCs may deviate from prototypic nonkeratinizing SCC, making them difficult to recognize. Presence of HPV-associated squamous dysplasia could serve as a morphologic clue.
PMID:39436498 | PMC:PMC11496466 | DOI:10.1007/s12105-024-01707-5
View details for PubMedID 39436498
-
More
-
Metabolic modulation of melanoma enhances the therapeutic potential of immune checkpoint inhibitors Frontiers in oncology
Gurel Z, Luy MS, Luo Q, Arp NL, Erbe AK, Kesarwala AH, Fan J, Kimple RJ
2024 Oct 1;14:1428802. doi: 10.3389/fonc.2024.1428802. eCollection 2024.
-
More
INTRODUCTION: Lactate is a pivotal molecule with diverse functions in the metabolic reprogramming of cancer cells. Beyond its role in metabolism, lactate exerts a modulatory effect within the tumor microenvironment; it is utilized by stromal cells and has been implicated in the suppression of the immune response against the tumor.
METHODS: Using in vitro assays (including flow cytometry, live-cell imaging and metabolic analyses), the impact of lactate dehydrogenase inhibitors (LDHIs) on melanoma cells were assessed. The therapeutic potential of LDHIs with immune checkpoint inhibitors (ICIs) were tested in vivo in murine models of melanoma tumors.
RESULTS: A potent anti-proliferative effect (via both cell cycle alterations and enhanced apoptosis) of LDHIs, Oxamate (Oxa) and methyl 1-hydroxy-6-phenyl-4-(trifluoromethyl)-1H-indole-2-carboxylate (NHI-2), was found upon treatment of melanoma cell lines. Using a combination of Oxa and NHI-2, a synergistic effect to inhibit proliferation, glycolysis, and ATP production was observed. Metabolic analysis revealed significant alteration in glycolysis and oxidative phosphorylation, while metabolite profiling emphasized consequential effects on lactate metabolism and induced energy depletion by LDHIs. Detection of increased RANTES and MCP-1, with Oxa and NHI-2 treatment, prompted the consideration of combining LDHIs with ICIs. In vivo studies using a murine B78 melanoma tumor model revealed a significant improvement in treatment efficacy when LDHIs were combined with ICIs.
CONCLUSIONS: These findings propose the potential of targeting lactate metabolism to enhance the efficacy of ICI treatments in patients with melanoma.
PMID:39435293 | PMC:PMC11491500 | DOI:10.3389/fonc.2024.1428802
View details for PubMedID 39435293
-
More
-
Challenges and opportunities for early phase clinical trials of novel drug-radiotherapy combinations: recommendations from NRG Oncology, the American Society for Radiation Oncology (ASTRO), the American College of Radiology (ACR), the Sarah Cannon Research Institute, and the American College of Radiation Oncology (ACRO) The Lancet. Oncology
Zumsteg ZS, Sheth S, Jabbour SK, Patel KR, Kimple RJ, Williams TM, Xu-Welliver M, Torres-Saavedra PA, Monjazeb AM, Mayadev J, Finkelstein SE, Buatti JM, Patel SP, Lin SH
2024 Oct;25(10):e489-e500. doi: 10.1016/S1470-2045(24)00264-X.
-
More
NRG Oncology's Developmental Therapeutics and Radiation Therapy Subcommittee assembled an interdisciplinary group of investigators to address barriers to successful early phase clinical trials of novel combination therapies involving radiation. This Policy Review elucidates some of the many challenges associated with study design for early phase trials combining radiotherapy with novel systemic agents, which are distinct from drug-drug combination development and are often overlooked. We also advocate for potential solutions that could mitigate or eliminate some of these barriers, providing examples of specific clinical trial designs that could help facilitate efficient and effective evaluation of novel drug-radiotherapy combinations.
PMID:39362260 | PMC:PMC11778933 | DOI:10.1016/S1470-2045(24)00264-X
View details for PubMedID 39362260
-
More
-
Chromosomal instability increases radiation sensitivity bioRxiv : the preprint server for biology
Cosper PF, Paracha M, Jones KM, Hrycyniak L, Henderson L, Bryan A, Eyzaguirre D, McCunn E, Boulanger E, Wan J, Nickel KP, Horner V, Hu R, Harari PM, Kimple RJ, Weaver BA
2024 Sep 19:2024.09.13.612942. doi: 10.1101/2024.09.13.612942.
-
More
Continuous chromosome missegregation over successive mitotic divisions, known as chromosomal instability (CIN), is common in cancer. Increasing CIN above a maximally tolerated threshold leads to cell death due to loss of essential chromosomes. Here, we show in two tissue contexts that otherwise isogenic cancer cells with higher levels of CIN are more sensitive to ionizing radiation, which itself induces CIN. CIN also sensitizes HPV-positive and HPV-negative head and neck cancer patient derived xenograft (PDX) tumors to radiation. Moreover, laryngeal cancers with higher CIN prior to treatment show improved response to radiation therapy. In addition, we reveal a novel mechanism of radiosensitization by docetaxel, a microtubule stabilizing drug commonly used in combination with radiation. Docetaxel causes cell death by inducing CIN due to abnormal multipolar spindles rather than causing mitotic arrest, as previously assumed. Docetaxel-induced CIN, rather than mitotic arrest, is responsible for the enhanced radiation sensitivity observed in vitro and in vivo, challenging the mechanistic dogma of the last 40 years. These results implicate CIN as a potential biomarker and inducer of radiation response, which could provide valuable cancer therapeutic opportunities.
STATEMENT OF SIGNIFICANCE: Cancer cells and laryngeal tumors with higher chromosome missegregation rates are more sensitive to radiation therapy, supporting chromosomal instability as a promising biomarker of radiation response.
PMID:39345631 | PMC:PMC11429890 | DOI:10.1101/2024.09.13.612942
View details for PubMedID 39345631
-
More
-
Targeted inhibition of BET proteins in HPV16-positive head and neck squamous cell carcinoma reveals heterogeneous transcriptional responses Frontiers in oncology
Rao A, Stosic MS, Mohanty C, Suresh D, Wang AR, Lee DL, Nickel KP, Chandrashekar DS, Kimple RJ, Lambert PF, Kendziorski C, Rounge TB, Iyer G
2024 Sep 5;14:1440836. doi: 10.3389/fonc.2024.1440836. eCollection 2024.
-
More
Human papillomaviruses (HPV), most commonly HPV16, are associated with a subset of head and neck squamous cell carcinoma (HNSCC) tumors, primarily oropharyngeal carcinomas, with integration of viral genomes into host chromosomes associated with worse survival outcomes. We analyzed TCGA data and found that HPV+ HNSCC expressed higher transcript levels of the bromodomain and extra terminal domain (BET) family of transcriptional coregulators. The role of BET protein-mediated transcription of viral-cellular genes in the viral-HNSCC genomes needs to be better understood. Using a combination of TAME-Seq, qRT-PCR, and immunoblot analyses, we show that BET inhibition downregulates E6 and E7 significantly, with heterogeneity in the downregulation of viral transcription across different HPV+ HNSCC cell lines. Chemical BET inhibition was phenocopied with the knockdown of BRD4, mirroring the downregulation of viral E6 and E7 expression. We found that BET inhibition directly downregulated c-Myc and E2F expression and induced CDKN1A (p21) expression, leading to a G1-cell cycle arrest with apoptotic activity. Overall, our studies demonstrate that BET inhibition regulates both E6 and E7 viral and key cellular cell cycle regulator E2F gene expression and cellular gene expression in HPV-associated HNSCC and highlight the potential of BET inhibitors as a therapeutic strategy for this disease while also underscoring the importance of considering the heterogeneity in cellular responses to BET inhibition.
PMID:39301555 | PMC:PMC11410754 | DOI:10.3389/fonc.2024.1440836
View details for PubMedID 39301555
-
More
-
Cancer therapy-related salivary dysfunction The Journal of clinical investigation
Paz C, Glassey A, Frick A, Sattar S, Zaorsky NG, Blitzer GC, Kimple RJ
2024 Sep 3;134(17):e182661. doi: 10.1172/JCI182661.
-
More
Salivary gland dysfunction is a common side effect of cancer treatments. Salivary function plays key roles in critical daily activities. Consequently, changes in salivary function can profoundly impair quality of life for cancer patients. We discuss salivary gland anatomy and physiology to understand how anticancer therapies such as chemotherapy, bone marrow transplantation, immunotherapy, and radiation therapy impair salivary function. We discuss approaches to quantify xerostomia in the clinic, including the advantages and limitations of validated quality-of-life instruments and approaches to directly measuring salivary function. Current and emerging approaches to treat cancer therapy-induced dry mouth are presented using radiation-induced salivary dysfunction as a model. Limitations of current sialagogues and salivary analogues are presented. Emerging approaches, including cellular and gene therapy and novel pharmacologic approaches, are described.
PMID:39225092 | PMC:PMC11364403 | DOI:10.1172/JCI182661
View details for PubMedID 39225092
-
More
-
Morphologic Spectrum of HPV-associated Sinonasal Carcinomas Head and neck pathology
Abi-Saab T, Lozar T, Chen Y, Tannenbaum AP, Geye H, Yu M, Weisman P, Harari PM, Kimple RJ, Lambert PF, Lloyd RV, Hu R
2024 Aug 5;18(1):67. doi: 10.1007/s12105-024-01670-1.
-
More
BACKGROUND: High-risk human papillomavirus (HR-HPV) infection has been increasingly recognized as a risk factor for sinonasal tract carcinomas. However the prevalence and prognostic significance of HPV-associated sinonasal carcinomas is not well known due to limited studies and inconsistency in HPV testing modalities in literatures. Morphologically, HPV-associated sinonasal carcinomas encompass a diverse group of tumors. HPV-associated sinonasal adenocarcinoma has not been reported. The purpose of this study was to determine the prevalence, morphologic spectrum and prognostic implication of HPV-associated sinonasal carcinomas.
METHODS: This cohort included 153 sinonasal carcinomas. Tissue microarrays were constructed. P16 immunohistochemistry and HR-HPV E6/7 in-situ Hybridization (ISH) were performed. Carcinomas were deemed HPV-associated based on a positive ISH testing. Clinicopathologic data was collected.
RESULTS: 28/153 (18%) sinonasal carcinomas were HPV-associated. HPV-associated carcinomas consisted of 26 (93%) squamous cell carcinomas and variants, 1 (3.5%) HPV-related multiphenotypic sinonasal carcinoma and 1 (3.5%) adenocarcinoma. The HPV-associated adenocarcinoma closely resembled HPV-associated endocervical adenocarcinoma morphologically. HPV-associated carcinomas occurred in 8 (29%) women and 20 (71%) men with a median age of 66 years old. HPV-associated carcinomas were predominantly located at nasal cavity. A trend toward improved overall survival and progression free survival in HPV-associated carcinomas patients was observed, yet without statistical significance.
CONCLUSION: Our study identifies a novel HPV-associated sinonasal adenocarcinoma subtype, highlights the broad morphologic spectrum of HPV-associated sinonasal carcinomas, and supports routine p16 testing during pathology practice regardless of tumor subtype followed by a confirmatory HR-HPV testing. This practice is critical for studying the clinical behavior of HPV-associated sinonasal carcinomas.
PMID:39101976 | PMC:PMC11300749 | DOI:10.1007/s12105-024-01670-1
View details for PubMedID 39101976
-
More
-
Genomic and Immune Landscape Comparison of MET Exon 14 Skipping and MET-Amplified Non-small Cell Lung Cancer Clinical lung cancer
Minne RL, Luo NY, Traynor AM, Huang M, DeTullio L, Godden J, Stoppler M, Kimple RJ, Baschnagel AM
2024 Sep;25(6):567-576.e1. doi: 10.1016/j.cllc.2024.05.001. Epub 2024 May 10.
-
More
BACKGROUND: Mutation or amplification of the mesenchymal-epithelial transition (MET) tyrosine kinase receptor causes dysregulation of receptor function and stimulates tumor growth in non-small cell lung cancer (NSCLC) with the most common mutation being MET exon 14 (METex14). We sought to compare the genomic and immune landscape of MET-altered NSCLC with MET wild-type NSCLC.
METHODS: 18,047 NSCLC tumors were sequenced with Tempus xT assay. Tumors were categorized based on MET exon 14 (METex14) mutations; low MET amplification defined as a copy number gain (CNG) 6-9, high MET amplification defined as CNG ≥ 10, and MET other type mutations. Immuno-oncology (IO) biomarkers and the frequency of other somatic gene alterations were compared across MET-altered and MET wild-type groups.
RESULTS: 276 (1.53%) METex14, 138 (0.76%) high METamp, 63 (0.35%) low METamp, 27 (0.15%) MET other, and 17,543 (97%) MET wild-type were identified. Patients with any MET mutation including METex14 were older, while patients with METex14 were more frequently female and nonsmokers. MET gene expression was highest in METamp tumors. PD-L1 positivity rates were higher in MET-altered groups than MET wild-type. METex14 exhibited the lowest tumor mutational burden (TMB) and lowest neoantigen tumor burden (NTB). METamp exhibited the lowest proportion of CD4 T cells and the highest proportion of NK cells. There were significant differences in co-alterations between METamp and METex14.
CONCLUSIONS: METex14 tumors exhibited differences in IO biomarkers and the somatic landscape compared to non-METex14 NSCLC tumors. Variations in immune profiles can affect immunotherapy selection in MET-altered NSCLC and require further exploration.
PMID:38852006 | PMC:PMC12121485 | DOI:10.1016/j.cllc.2024.05.001
View details for PubMedID 38852006
-
More
-
Multidisciplinary Management of Advanced Thyroid Cancer JCO oncology practice
Bruce JY, Glazer TA, Kimple RJ
2024 Jul;20(7):877-878. doi: 10.1200/OP.24.00283. Epub 2024 Mar 29.
-
More
Bruce, Glazer, and Kimple discuss advances in the management of advanced thyroid carcinoma and the role of surgery and radiation to provide context to the review by Yun and Cohen focused on systemic therapy.
PMID:38810182 | PMC:PMC11451261 | DOI:10.1200/OP.24.00283
View details for PubMedID 38810182
-
More
-
An algorithm for standardization of tumor Infiltrating lymphocyte evaluation in head and neck cancers Oral oncology
Xirou V, Moutafi M, Bai Y, Aung TN, Burela S, Liu M, Kimple RJ, Ahmed FS, Schultz B, Flieder D, Connolly DC, Psyrri A, Burtness B, Rimm DL
2024 May;152:106750. doi: 10.1016/j.oraloncology.2024.106750. Epub 2024 Mar 27.
-
More
PURPOSE: The prognostic and predictive significance of pathologist-read tumor infiltrating lymphocytes (TILs) in head and neck cancers have been demonstrated through multiple studies over the years. TILs have not been broadly adopted clinically, perhaps due to substantial inter-observer variability. In this study, we developed a machine-based algorithm for TIL evaluation in head and neck cancers and validated its prognostic value in independent cohorts.
EXPERIMENTAL DESIGN: A network classifier called NN3-17 was trained to identify and calculate tumor cells, lymphocytes, fibroblasts and "other" cells on hematoxylin-eosin stained sections using the QuPath software. These measurements were used to construct three predefined TIL variables. A retrospective collection of 154 head and neck squamous cell cancer cases was used as the discovery set to identify optimal association of TIL variables and survival. Two independent cohorts of 234 cases were used for validation.
RESULTS: We found that electronic TIL variables were associated with favorable prognosis in both the HPV-positive and -negative cases. After adjusting for clinicopathologic factors, Cox regression analysis demonstrated that electronic total TILs% (p = 0.025) in the HPV-positive and electronic stromal TILs% (p < 0.001) in the HPV-negative population were independent markers of disease specific outcomes (disease free survival).
CONCLUSIONS: Neural network TIL variables demonstrated independent prognostic value in validation cohorts of HPV-positive and HPV-negative head and neck cancers. These objective variables can be calculated by an open-source software and could be considered for testing in a prospective setting to assess potential clinical implications.
PMID:38547779 | PMC:PMC11060915 | DOI:10.1016/j.oraloncology.2024.106750
View details for PubMedID 38547779
-
More
-
Development of an Engineered Single-Domain Antibody for Targeting MET in Non-Small Cell Lung Cancer Bioconjugate chemistry
Luo NY, Minne RL, Gallant JP, Gunaratne GS, West JL, Javeri S, Robertson AJ, Lake EW, Engle JW, Mixdorf JC, Aluicio-Sarduy E, Nickel KP, Hernandez R, Kimple RJ, Baschnagel AM, LeBeau AM
2024 Mar 20;35(3):389-399. doi: 10.1021/acs.bioconjchem.4c00019. Epub 2024 Mar 12.
-
More
The Mesenchymal Epithelial Transition (MET) receptor tyrosine kinase is upregulated or mutated in 5% of non-small-cell lung cancer (NSCLC) patients and overexpressed in multiple other cancers. We sought to develop a novel single-domain camelid antibody with high affinity for MET that could be used to deliver conjugated payloads to MET expressing cancers. From a naïve camelid variable-heavy-heavy (VHH) domain phage display library, we identified a VHH clone termed 1E7 that displayed high affinity for human MET and was cross-reactive with MET across multiple species. When expressed as a bivalent human Fc fusion protein, 1E7-Fc was found to selectively bind to EBC-1 (MET amplified) and UW-Lung 21 (MET exon 14 mutated) cell lines by flow cytometry and immunofluorescence imaging. Next, we investigated the ability of [89Zr]Zr-1E7-Fc to detect MET expression in vivo by PET/CT imaging. [89Zr]Zr-1E7-Fc demonstrated rapid localization and high tumor uptake in both xenografts with a %ID/g of 6.4 and 5.8 for EBC-1 and UW-Lung 21 at 24 h, respectively. At the 24 h time point, clearance from secondary and nontarget tissues was also observed. Altogether, our data suggest that 1E7-Fc represents a platform technology that can be employed to potentially both image and treat MET-altered NSCLC.
PMID:38470611 | PMC:PMC12060584 | DOI:10.1021/acs.bioconjchem.4c00019
View details for PubMedID 38470611
-
More
-
A Phase 2 Randomized Clinical Trial Evaluating 4-Dimensional Computed Tomography Ventilation-Based Functional Lung Avoidance Radiation Therapy for Non-Small Cell Lung Cancer International journal of radiation oncology, biology, physics
Baschnagel AM, Flakus MJ, Wallat EM, Wuschner AE, Chappell RJ, Bayliss RA, Kimple RJ, Christensen GE, Reinhardt JM, Bassetti MF, Bayouth JE
2024 Aug 1;119(5):1393-1402. doi: 10.1016/j.ijrobp.2024.02.019. Epub 2024 Feb 20.
-
More
PURPOSE: To determine whether 4-dimensional computed tomography (4DCT) ventilation-based functional lung avoidance radiation therapy preserves pulmonary function compared with standard radiation therapy for non-small cell lung cancer (NSCLC).
METHODS AND MATERIALS: This single center, randomized, phase 2 trial enrolled patients with NSCLC receiving curative intent radiation therapy with either stereotactic body radiation therapy or conventionally fractionated radiation therapy between 2016 and 2022. Patients were randomized 1:1 to standard of care radiation therapy or functional lung avoidance radiation therapy. The primary endpoint was the change in Jacobian-based ventilation as measured on 4DCT from baseline to 3 months postradiation. Secondary endpoints included changes in volume of high- and low-ventilating lung, pulmonary toxicity, and changes in pulmonary function tests (PFTs).
RESULTS: A total of 122 patients were randomized and 116 were available for analysis. Median follow up was 29.9 months. Functional avoidance plans significantly (P < .05) reduced dose to high-functioning lung without compromising target coverage or organs at risk constraints. When analyzing all patients, there was no difference in the amount of lung showing a reduction in ventilation from baseline to 3 months between the 2 arms (1.91% vs 1.87%; P = .90). Overall grade ≥2 and grade ≥3 pulmonary toxicities for all patients were 24.1% and 8.6%, respectively. There was no significant difference in pulmonary toxicity or changes in PFTs between the 2 study arms. In the conventionally fractionated cohort, there was a lower rate of grade ≥2 pneumonitis (8.2% vs 32.3%; P = .049) and less of a decline in change in forced expiratory volume in 1 second (-3 vs -5; P = .042) and forced vital capacity (1.5 vs -6; P = .005) at 3 months, favoring the functional avoidance arm.
CONCLUSIONS: There was no difference in posttreatment ventilation as measured by 4DCT between the arms. In the cohort of patients treated with conventionally fractionated radiation therapy with functional lung avoidance, there was reduced pulmonary toxicity, and less decline in PFTs suggesting a clinical benefit in patients with locally advanced NSCLC.
PMID:38387810 | DOI:10.1016/j.ijrobp.2024.02.019
View details for PubMedID 38387810
-
More
-
Network analyses: Inhibition of androgen receptor signaling reduces inflammation in the lung through AR-MAF-IL6 signaling axes Genes & diseases
Wang AR, Baschnagel AM, Ni Z, Brennan SR, Newton HK, Buehler D, Kendziorski C, Kimple RJ, Iyer G
2023 Aug 18;11(3):101072. doi: 10.1016/j.gendis.2023.07.001. eCollection 2024 May.
-
More
PMID:38292196 | PMC:PMC10825295 | DOI:10.1016/j.gendis.2023.07.001
View details for PubMedID 38292196
-
More
-
Functionality of bone marrow mesenchymal stromal cells derived from head and neck cancer patients - A FDA-IND enabling study regarding MSC-based treatments for radiation-induced xerostomia Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Blitzer GC, Paz C, Glassey A, Ganz OR, Giri J, Pennati A, Meyers RO, Bates AM, Nickel KP, Weiss M, Morris ZS, Mattison RJ, McDowell KA, Croxford E, Chappell RJ, Glazer TA, Rogus-Pulia NM, Galipeau J, Kimple RJ
2024 Mar;192:110093. doi: 10.1016/j.radonc.2024.110093. Epub 2024 Jan 13.
-
More
PURPOSE: Salivary dysfunction is a significant side effect of radiation therapy for head and neck cancer (HNC). Preliminary data suggests that mesenchymal stromal cells (MSCs) can improve salivary function. Whether MSCs from HNC patients who have completed chemoradiation are functionally similar to those from healthy patients is unknown. We performed a pilot clinical study to determine whether bone marrow-derived MSCs [MSC(M)] from HNC patients could be used for the treatment of RT-induced salivary dysfunction.
METHODS: An IRB-approved pilot clinical study was undertaken on HNC patients with xerostomia who had completed treatment two or more years prior. Patients underwent iliac crest bone marrow aspirate and MSC(M) were isolated and cultured. Culture-expanded MSC(M) were stimulated with IFNγ and cryopreserved prior to reanimation and profiling for functional markers by flow cytometry and ELISA. MSC(M) were additionally injected into mice with radiation-induced xerostomia and the changes in salivary gland histology and salivary production were examined.
RESULTS: A total of six subjects were enrolled. MSC(M) from all subjects were culture expanded to > 20 million cells in a median of 15.5 days (range 8-20 days). Flow cytometry confirmed that cultured cells from HNC patients were MSC(M). Functional flow cytometry demonstrated that these IFNγ-stimulated MSC(M) acquired an immunosuppressive phenotype. IFNγ-stimulated MSC(M) from HNC patients were found to express GDNF, WNT1, and R-spondin 1 as well as pro-angiogenesis and immunomodulatory cytokines. In mice, IFNγ-stimulated MSC(M) injection after radiation decreased the loss of acinar cells, decreased the formation of fibrosis, and increased salivary production.
CONCLUSIONS: MSC (M) from previously treated HNC patients can be expanded for auto-transplantation and are functionally active. Furthermore IFNγ-stimulated MSC(M) express proteins implicated in salivary gland regeneration. This study provides preliminary data supporting the feasibility of using autologous MSC(M) from HNC patients to treat RT-induced salivary dysfunction.
PMID:38224919 | PMC:PMC10922976 | DOI:10.1016/j.radonc.2024.110093
View details for PubMedID 38224919
-
More
-
MET Inhibitor Capmatinib Radiosensitizes MET Exon 14-Mutated and MET-Amplified Non-Small Cell Lung Cancer International journal of radiation oncology, biology, physics
Ramesh S, Cifci A, Javeri S, Minne RL, Longhurst CA, Nickel KP, Kimple RJ, Baschnagel AM
2024 Apr 1;118(5):1379-1390. doi: 10.1016/j.ijrobp.2023.11.013. Epub 2023 Nov 16.
-
More
PURPOSE: The objective of this study was to investigate the effects of inhibiting the MET receptor with capmatinib, a potent and clinically relevant ATP-competitive tyrosine kinase inhibitor, in combination with radiation in MET exon 14-mutated and MET-amplified non-small cell lung (NSCLC) cancer models.
METHODS AND MATERIALS: In vitro effects of capmatinib and radiation on cell proliferation, colony formation, MET signaling, apoptosis, and DNA damage repair were evaluated. In vivo tumor responses were assessed in cell line xenograft and patient-derived xenograft models. Immunohistochemistry was used to confirm the in vitro results.
RESULTS: In vitro clonogenic survival assays demonstrated radiosensitization with capmatinib in both MET exon 14-mutated and MET-amplified NSCLC cell lines. No radiation-enhancing effect was observed in MET wild-type NSCLC and a human bronchial epithelial cell line. Minimal apoptosis was detected with the combination of capmatinib and radiation. Capmatinib plus radiation compared with radiation alone resulted in inhibition of DNA double-strand break repair, as measured by prolonged expression of γH2AX. In vivo, the combination of capmatinib and radiation significantly delayed tumor growth compared with vehicle control, capmatinib alone, or radiation alone. Immunohistochemistry indicated inhibition of phospho-MET and phospho-S6 and a decrease in Ki67 with inhibition of MET.
CONCLUSIONS: Inhibition of MET with capmatinib enhances the effect of radiation in both MET exon 14-mutated and MET-amplified NSCLC models.
PMID:37979706 | PMC:PMC12121486 | DOI:10.1016/j.ijrobp.2023.11.013
View details for PubMedID 37979706
-
More
-
MET Inhibitor Capmatinib Radiosensitizes MET Exon 14-Mutated and MET-Amplified Non-Small Cell Lung Cancer bioRxiv : the preprint server for biology
Ramesh S, Cifci A, Javeri S, Minne R, Longhurst CA, Nickel KP, Kimple RJ, Baschnagel AM
2023 Oct 27:2023.10.26.564232. doi: 10.1101/2023.10.26.564232.
-
More
PURPOSE: The objective of this study was to investigate the effects of inhibiting the MET receptor with capmatinib, a potent and clinically relevant ATP-competitive tyrosine kinase inhibitor, in combination with radiation in MET exon 14-mutated and MET-amplified non-small cell lung (NSCLC) cancer models.
METHODS AND MATERIALS: In vitro effects of capmatinib and radiation on cell proliferation, colony formation, MET signaling, apoptosis, and DNA damage repair were evaluated. In vivo tumor responses were assessed in cell line xenograft and patient-derived xenograft models. Immunohistochemistry (IHC) was used to confirm in vitro results.
RESULTS: In vitro clonogenic survival assays demonstrated radiosensitization with capmatinib in both MET exon 14-mutated and MET-amplified NSCLC cell lines. No radiation-enhancing effect was observed in MET wild-type NSCLC and human bronchial epithelial cell line. Minimal apoptosis was detected with the combination of capmatinib and radiation. Capmatinib plus radiation compared to radiation alone resulted in inhibition of DNA double-strand break repair as measured by prolonged expression of γH2AX. In vivo, the combination of capmatinib and radiation significantly delayed tumor growth compared to vehicle control, capmatinib alone, or radiation alone. IHC indicated inhibition of phospho-MET and phospho-S6 and a decrease in Ki67 with inhibition of MET.
CONCLUSIONS: Inhibition of MET with capmatinib enhanced the effect of radiation in both MET exon 14-mutated and MET-amplified NSCLC models.
PMID:37961176 | PMC:PMC10634863 | DOI:10.1101/2023.10.26.564232
View details for PubMedID 37961176
-
More
-
Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): A pilot, first-in-human study of interferon gamma-stimulated marrow mesenchymal stromal cells for treatment of radiation-induced xerostomia Cytotherapy
Blitzer GC, Glazer T, Burr A, Gustafson S, Ganz O, Meyers R, McDowell KA, Nickel KP, Mattison RJ, Weiss M, Chappell R, Rogus-Pulia NM, Galipeau J, Kimple RJ
2023 Nov;25(11):1139-1144. doi: 10.1016/j.jcyt.2023.07.009. Epub 2023 Aug 15.
-
More
BACKGROUND AIMS: Xerostomia, or the feeling of dry mouth, is a significant side effect of radiation therapy for patients with head and neck cancer (HNC). Preliminary data suggest that mesenchymal stromal/stem cells (MSCs) can improve salivary function. We performed a first-in-human pilot study of interferon gamma (IFNγ)-stimulated autologous bone marrow-derived MSCs, or MSC(M), for the treatment of radiation-induced xerostomia (RIX). Here we present the primary safety and secondary efficacy endpoints.
METHODS: A single-center pilot clinical trial was conducted investigating the safety and tolerability of autologous IFNγ-stimulated MSC(M). The study was conducted under an approved Food and Drug Administration Investigational New Drug application using an institutional review board-approved protocol (NCT04489732). Patients underwent iliac crest bone marrow aspirate and MSC(M) were isolated, cultured, stimulated with IFNγ and cryopreserved for later use. Banked cells were thawed and allowed to recover in culture before patients received a single injection of 10 × 106 MSC(M) into the right submandibular gland under ultrasound guidance. The primary objective was determination of safety and tolerability by evaluating dose-limiting toxicity (DLT). A DLT was defined as submandibular pain >5 on a standard 10-point pain scale or any serious adverse event (SAE) within 1 month after injection. Secondary objectives included analysis of efficacy as measured by salivary quantification and using three validated quality of life instruments. Quantitative results are reported as mean and standard deviation.
RESULTS: Six patients with radiation-induced xerostomia who had completed radiation at least 2 years previously (average 7.8 years previously) were enrolled in the pilot study. The median age was 71 (61-74) years. Five (83%) patients were male. Five patients (83%) were treated with chemoradiation and one patient (17%) with radiation alone. Grade 1 pain was seen in 50% of patients after submandibular gland injection; all pain resolved within 4 days. No patients reported pain 1 month after injection, with no SAE or other DLTs reported 1 month after injection. The analysis of secondary endpoints demonstrated a trend of increased salivary production. Three patients (50%) had an increase in unstimulated saliva at 1 and 3 months after MSC(M) injection. Quality of life surveys also showed a trend toward improvement.
CONCLUSIONS: Injection of autologous IFNγ-stimulated MSC(M) into a singular submandibular gland of patients with RIX is safe and well tolerated in this pilot study. A trend toward an improvement in secondary endpoints of salivary quantity and quality of life was observed. This first-in-human study provides support for further investigation into IFNγ-stimulated MSC(M) injected in both submandibular glands as an innovative approach to treat RIX and improve quality of life for patients with HNC.
PMID:37589639 | PMC:PMC10615723 | DOI:10.1016/j.jcyt.2023.07.009
View details for PubMedID 37589639
-
More
-
Radiation Sensitivity: The Rise of Predictive Patient-Derived Cancer Models Seminars in radiation oncology
Berube LL, Nickel KP, Iida M, Ramisetty S, Kulkarni P, Salgia R, Wheeler DL, Kimple RJ
2023 Jul;33(3):279-286. doi: 10.1016/j.semradonc.2023.03.005.
-
More
Patient-derived cancer models have been used for decades to improve our understanding of cancer and test anticancer treatments. Advances in radiation delivery have made these models more attractive for studying radiation sensitizers and understanding an individual patient's radiation sensitivity. Advances in the use of patient-derived cancer models lead to a more clinically relevant outcome, although many questions remain regarding the optimal use of patient-derived xenografts and patient-derived spheroid cultures. The use of patient-derived cancer models as personalized predictive avatars through mouse and zebrafish models is discussed, and the advantages and disadvantages of patient-derived spheroids are reviewed. In addition, the use of large repositories of patient-derived models to develop predictive algorithms to guide treatment selection is discussed. Finally, we review methods for establishing patient-derived models and identify key factors that influence their use as both avatars and models of cancer biology.
PMID:37331782 | PMC:PMC10287034 | DOI:10.1016/j.semradonc.2023.03.005
View details for PubMedID 37331782
-
More
-
Circulating tumor cell abundance in head and neck squamous cell carcinoma decreases with successful chemoradiation and cetuximab treatment Cancer letters
Poellmann MJ, Bu J, Kim D, Iida M, Hong H, Wang AZ, Wheeler DL, Kimple RJ, Hong S
2023 May 28;562:216187. doi: 10.1016/j.canlet.2023.216187. Epub 2023 Apr 15.
-
More
Head and neck squamous cell carcinoma (HNSCC) is a common and deadly cancer. Circulating tumor cell (CTC) abundance may a valuable, prognostic biomarker in low- and intermediate-risk patients. However, few technologies have demonstrated success in detecting CTCs in these populations. We prospectively collected longitudinal CTC counts from two cohorts of patients receiving treatments at our institution using a highly sensitive device that purifies CTCs using biomimetic cell rolling and dendrimer-conjugated antibodies. In patients with intermediate risk human papillomavirus (HPV)-positive HNSCC, elevated CTC counts were detected in 13 of 14 subjects at screening with a median of 17 CTC/ml (range 0.2-2986.5). A second cohort of non-metastatic, HPV- HNSCC subjects received cetuximab monotherapy followed by surgical resection. In this cohort, all subjects had elevated baseline CTC counts median of 73 CTC/ml (range 5.4-332.9) with statistically significant declines during treatment. Interestingly, two patients with recurrent disease had elevated CTC counts during and following treatment, which also correlated with growth of size and ki67 expression in the primary tumor. The results suggest that our device may be a valuable tool for evaluating the success of less intensive treatment regimens.
PMID:37068555 | PMC:PMC10510654 | DOI:10.1016/j.canlet.2023.216187
View details for PubMedID 37068555
-
More
-
Biological Characterization of the Effects of Filtration on the Xoft Axxent® Electronic Brachytherapy Source for Cervical Cancer Applications Radiation research
Walter AE, Cosper PF, Nickel KP, Ramesh S, Khan AU, DeWerd LA, Kimple RJ
2023 May 1;199(5):429-438. doi: 10.1667/RADE-22-00112.1.
-
More
Low-energy X-ray sources that operate in the kilovoltage energy range have been shown to induce more cellular damage when compared to their megavoltage counterparts. However, low-energy X-ray sources are more susceptible to the effects of filtration on the beam spectrum. This work sought to characterize the biological effects of the Xoft Axxent® source, a low-energy therapeutic X-ray source, both with and without the titanium vaginal applicator in place. It was hypothesized that there would be an increase in relative biological effectiveness (RBE) of the Axxent® source compared to 60Co and that the source in the titanium vaginal applicator (SIA) would have decreased biological effects compared to the bare source (BS). This hypothesis was drawn from linear energy transfer (LET) simulations performed using the TOPAS Monte Carlo user code as well a reduction in dose rate of the SIA compared to the BS. A HeLa cell line was maintained and used to evaluate these effects. Clonogenic survival assays were performed to evaluate differences in the RBE between the BS and SIA using 60Co as the reference beam quality. Neutral comet assay was used to assess induction of DNA strand damage by each beam to estimate differences in RBE. Quantification of mitotic errors was used to evaluate differences in chromosomal instability (CIN) induced by the three beam qualities. The BS was responsible for the greatest quantity of cell death due to a greater number of DNA double strand breaks (DSB) and CIN observed in the cells. The differences observed in the BS and SIA surviving fractions and RBE values were consistent with the 13% difference in LET as well as the factor of 3.5 reduction in dose rate of the SIA. Results from the comet and CIN assays were consistent with these results as well. The use of the titanium applicator results in a reduction in the biological effects observed with these sources, but still provides an advantage over megavoltage beam qualities. © 2023 by Radiation Research Society.
PMID:37014873 | PMC:PMC10288372 | DOI:10.1667/RADE-22-00112.1
View details for PubMedID 37014873
-
More
-
HPV16 E6 induces chromosomal instability due to polar chromosomes caused by E6AP-dependent degradation of the mitotic kinesin CENP-E Proceedings of the National Academy of Sciences of the United States of America
Cosper PF, Hrycyniak CF, Paracha M, Lee DL, Wan J, Jones K, Bice SA, Nickel K, Mallick S, Taylor AM, Kimple RJ, Lambert PF, Weaver BA
2023 Apr 4;120(14):e2216700120. doi: 10.1073/pnas.2216700120. Epub 2023 Mar 29.
-
More
Chromosome segregation during mitosis is highly regulated to ensure production of genetically identical progeny. Recurrent mitotic errors cause chromosomal instability (CIN), a hallmark of tumors. The E6 and E7 oncoproteins of high-risk human papillomavirus (HPV), which causes cervical, anal, and head and neck cancers (HNC), cause mitotic defects consistent with CIN in models of anogenital cancers, but this has not been studied in the context of HNC. Here, we show that HPV16 induces a specific type of CIN in patient HNC tumors, patient-derived xenografts, and cell lines, which is due to defects in chromosome congression. These defects are specifically induced by the HPV16 oncogene E6 rather than E7. We show that HPV16 E6 expression causes degradation of the mitotic kinesin CENP-E, whose depletion produces chromosomes that are chronically misaligned near spindle poles (polar chromosomes) and fail to congress. Though the canonical oncogenic role of E6 is the degradation of the tumor suppressor p53, CENP-E degradation and polar chromosomes occur independently of p53. Instead, E6 directs CENP-E degradation in a proteasome-dependent manner via the E6-associated ubiquitin protein ligase E6AP/UBE3A. This study reveals a mechanism by which HPV induces CIN, which may impact HPV-mediated tumor initiation, progression, and therapeutic response.
PMID:36989302 | PMC:PMC10083562 | DOI:10.1073/pnas.2216700120
View details for PubMedID 36989302
-
More
-
Inhibiting IGF1R-mediated Survival Signaling in Head and Neck Cancer with the Peptidomimetic SSTN<sub>IGF1R</sub> Cancer research communications
Stueven NA, Beauvais DM, Hu R, Kimple RJ, Rapraeger AC
2023 Jan 19;3(1):97-108. doi: 10.1158/2767-9764.CRC-22-0274. eCollection 2023 Jan.
-
More
Previous studies have shown that the type I IGFR (IGF1R) suppresses apoptosis when it is autoactivated by coupling its extracellular domain to a matrix adhesion receptor complex consisting of syndecan-1 (Sdc1) and αvβ3 or αvβ5 integrin. We now report that head and neck squamous cell carcinoma (HNSCC) relies on this receptor complex. Disruption of the complex in HNSCC cells in vitro with a peptide mimetic of the organizer site in Sdc1 (called SSTNIGF1R) inactivates IGF1R, even in the presence of IGF1, and relieves the suppression of apoptosis signal-regulating kinase-1 (ASK1), dramatically reducing tumor cell survival. Normal epithelial cells do not assemble this receptor complex, require IGF1 to activate the IGF1R, and are refractory to SSTNIGF1R. In vivo, SSTNIGF1R reduced the growth of patient-derived HNSCC tumors in immunodeficient mice by 85%-95%. IGF1R's assimilation into the matrix receptor complex, which is detected in these tumors using the proximity ligation assay (PLA), is quantitatively disrupted by SSTNIGF1R, coinciding with ASK1 activation. PLA also detects the IGF1R-containing receptor complex in the archival sections of tonsil carcinomas, whereas the adjacent benign epithelium is negative. Likewise, PLA screening of oropharyngeal and adenoid cystic tumor microarrays demonstrated that over 95% of the tumors contained this unique receptor complex with no detectable expression in benign tissue. These findings suggest that HNSCC upregulates and is highly dependent on IGF1R signaling via this adhesion receptor complex. Targeting this mechanism with novel therapeutics, including highly specific SSTNIGF1R, is likely to offer promising outcomes for patients with carcinoma.
SIGNIFICANCE: A newly developed biomarker reveals upregulation of an antiapoptotic IGF1R-integrin-syndecan receptor complex in head and neck cancer and documents disruption of the complex in patient-derived tumor xenografts (PDX) treated with the inhibitor SSTNIGF1R. A corresponding blockade in PDX growth in the presence of this inhibitor demonstrates that therapies designed to target this mechanism will likely offer promising outcomes for patients with head and neck cancer.
PMID:36968227 | PMC:PMC10035507 | DOI:10.1158/2767-9764.CRC-22-0274
View details for PubMedID 36968227
-
More
-
Interstitial Brachytherapy for Lip Cancer: Technical Aspects to Individualize Treatment Approach and Optimize Outcomes Practical radiation oncology
Merfeld EC, Witek ME, Francis DM, Burr AR, Wallace CR, Kuczmarska-Haas A, Lamichhane N, Kimple RJ, Glazer TA, Wieland AM, McCulloch TM, Hartig GK, Harari PM
2023 Jul-Aug;13(4):340-345. doi: 10.1016/j.prro.2023.01.004. Epub 2023 Jan 25.
-
More
Primary radiation therapy using interstitial brachytherapy (IBT) provides excellent local tumor control for early-stage squamous cell carcinoma of the lip. Technical aspects of treatment are important to optimize outcomes. In this report, we discuss patient selection criteria, procedural details, and dosimetric considerations for performing IBT for cancers of the lip. Catheters are inserted across the length of tumor entering and exiting approximately 5 mm beyond the palpable tumor extent. A custom mouthpiece is fabricated to facilitate normal tissue sparing. Patients undergo computed tomography imaging, the gross tumor volume is contoured based on physical examination and computed tomography findings, and an individualized brachytherapy plan is generated with the goals of achieving gross tumor volume D90% ≥ 90% and minimizing V150%. Ten patients with primary (n = 8) or recurrent (n = 2) cancers of the lip who received high-dose-rate lip IBT using 2.0- to 2.5-week treatment regimens are described (median prescription: 47.6 Gy in 14 fractions of 3.4 Gy). Local tumor control was 100%. There were no cases of acute grade ≥4 or late grade ≥2 toxicity, and cosmesis scores were graded as good to excellent in all patients. IBT represents an excellent treatment option for patients with lip squamous cell carcinoma. With careful attention to technical considerations furthered described in the present report, high rates of tumor control, low rates of toxicity, and favorable esthetic and functional outcomes can be achieved with IBT for lip cancer.
PMID:36709044 | PMC:PMC10330101 | DOI:10.1016/j.prro.2023.01.004
View details for PubMedID 36709044
-
More
-
Patterns of failure for hypopharynx cancer patients treated with limited high-dose radiotherapy treatment volumes Radiation oncology journal
Burr A, Harari P, Wieland A, Kimple R, Hartig G, Witek M
2022 Dec;40(4):225-231. doi: 10.3857/roj.2022.00311. Epub 2022 Dec 2.
-
More
PURPOSE: Optimal radiotherapy treatment volumes for patients with locally advanced hypopharynx squamous cell carcinoma should ensure maximal tumor coverage with minimal inclusion of normal surrounding structures. Here we evaluated the effectiveness of a direct 3-mm high-dose gross tumor volume to planning target volume expansion on clinical outcomes for hypopharynx cancers.
MATERIALS AND METHODS: We performed a retrospective analysis of patients with hypopharynx carcinoma treated between 2004 and 2018 with primary radiotherapy using a direct high-dose gross tumor volume to planning target volume expansion and with or without concurrent systemic therapy. Diagnostic imaging of recurrences was co-registered with the planning CT. Spatial and volumetric analyses of contoured recurrences were compared with planned isodose lines. Failures were initially defined as in field, marginal, elective nodal, and out of field. Each failure was further classified as central high-dose, peripheral high-dose, central intermediate/low-dose, peripheral intermediate/low-dose, and extraneous. Clinical outcomes were analyzed by Kaplan-Meier estimation.
RESULTS: Thirty-six patients were identified. At a median follow-up at 52.4 months, estimated 5-year overall survival was 59.3% (95% confidence interval [CI], 36.3%-74.1%), 5-year local and nodal control was 71.7% (95% CI, 47.1%-86.3%) and 69.9% (95% CI, 57.0%-82.6%), respectively. The most common failure was in the high-dose primary target volume. The gastrostomy tube retention rate at 1 year among patients without recurrence was 13.0% (95% CI, 3.2%-29.7%).
CONCLUSION: Minimal high-dose target volume expansions for hypopharynx cancers were associated with favorable locoregional control. This approach may enable therapy intensification to improve clinical outcomes.
PMID:36456541 | PMC:PMC9830040 | DOI:10.3857/roj.2022.00311
View details for PubMedID 36456541
-
More
-
Safety of Nivolumab Added to Chemoradiation Therapy Platforms for Intermediate and High-Risk Locoregionally Advanced Head and Neck Squamous Cell Carcinoma: RTOG Foundation 3504 International journal of radiation oncology, biology, physics
Gillison ML, Ferris RL, Harris J, Colevas AD, Mell LK, Kong C, Jordan RC, Moore KL, Truong M, Kirsch C, Chakravarti A, Blakaj DM, Clump DA, Ohr JP, Deeken JF, Gensheimer MF, Saba NF, Dorth JA, Rosenthal DI, Leidner RS, Kimple RJ, Machtay M, Curran WJ, Torres-Saavedra P, Le QT
2023 Mar 15;115(4):847-860. doi: 10.1016/j.ijrobp.2022.10.008. Epub 2022 Oct 11.
-
More
PURPOSE: Programmed death-1 immune checkpoint blockade improves survival of patients with recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), but the benefits of addition to (chemo)radiation for newly diagnosed patients with HNSCC remain unknown.
METHODS AND MATERIALS: We evaluated the safety of nivolumab concomitant with 70 Gy intensity modulated radiation therapy and weekly cisplatin (arm 1), every 3-week cisplatin (arm 2), cetuximab (arm 3), or alone for platinum-ineligible patients (arm 4) in newly diagnosed intermediate- or high-risk locoregionally advanced HNSCC. Patients received nivolumab from 2 weeks prior to radiation therapy until 3 months post-radiation therapy. The primary endpoint was dose-limiting toxicity (DLT). If ≤2 of the first 8 evaluable patients experienced a DLT, an arm was considered safe. Secondary endpoints included toxicity and feasibility of adjuvant nivolumab to 1 year, defined as all 7 additional doses received by ≥4 of the first 8 evaluable patients across arms.
RESULTS: Of 39 patients (10 in arms 1, 3, 4 and 9 in arm 2), 72% had T3-4 tumors, 85% had N2-3 nodal disease, and 67% had >10 pack-years of smoking. There were no DLTs in arms 1 and 2, 1 in arm 3 (mucositis), and 2 in arm 4 (lipase elevation and mucositis in 1 and fatigue in another). The most common grade ≥3 nivolumab-related adverse events were lipase increase, mucositis, diarrhea, lymphopenia, hyponatremia, leukopenia, fatigue, and serum amylase increase. Adjuvant nivolumab was feasible as defined in the protocol.
CONCLUSIONS: Concomitant nivolumab with the 4 tested regimens was safe for patients with intermediate- and high-risk HNSCC, and subsequent adjuvant nivolumab was feasible as defined (NCT02764593).
PMID:36228746 | PMC:PMC11189668 | DOI:10.1016/j.ijrobp.2022.10.008
View details for PubMedID 36228746
-
More
-
Quantification of very late xerostomia in head and neck cancer patients after irradiation Laryngoscope investigative otolaryngology
Blitzer GC, Rogus-Pulia NM, Paz C, Nickel KP, Cannaday VL, Kelm-Nelson CA, Sudakaran S, Chappell RJ, Glazer T, Kimple RJ
2022 Jul 12;7(4):1018-1024. doi: 10.1002/lio2.864. eCollection 2022 Aug.
-
More
OBJECTIVE: Radiation therapy (RT) for head and neck cancer (HNC) can result in severe xerostomia, or the subjective feeling of dry mouth. Characterizing xerostomia is critical to designing future clinical trials investigating how to improve HNC patients' quality of life (QoL). Few studies have investigated the very late (>5 years post-RT) effects of RT for HNC. We undertook preliminary studies quantifying very late xerostomia.
METHODS: Six adults who underwent RT for HNC at least 5 years prior and reported xerostomia were enrolled. Five healthy adults without a self-reported history of HNC or xerostomia were enrolled as controls. All participants completed three validated surveys to measure xerostomia-related QoL. Salivary production rates were measured and compositional analysis of the saliva and oral microbiome was completed.
RESULTS: The QoL survey scores for the HNC participants were significantly worse as compared to the control participants. The HNC participants produced less unstimulated saliva (p = .02) but not less stimulated saliva. The median salivary mucin significantly higher in HNC participants than in control participants (p = .02). There was no significant difference between the pH, amylase, or total protein. Microbiome analysis revealed alpha diversity to be significantly lower in the HNC participants.
CONCLUSION: In the survivors of HNC who suffer from late toxicities, multiple means of measuring toxicity may be useful. We found that in patients with radiation-induced xerostomia over 5 years after therapy, not only were the QoL surveys significantly worse, as expected, but other measurements such as mucin and oral microbiome diversity were also significantly different.
LEVEL OF EVIDENCE: 3.
PMID:36000048 | PMC:PMC9392383 | DOI:10.1002/lio2.864
View details for PubMedID 36000048
-
More
-
Bimodal liquid biopsy for cancer immunotherapy based on peptide engineering and nanoscale analysis Biosensors & bioelectronics
Bu J, Jeong W, Jafari R, Kubiatowicz LJ, Nair A, Poellmann MJ, Hong RS, Liu EW, Owen RH, Rawding PA, Hopkins CM, Kim D, George DJ, Armstrong AJ, Král P, Wang AZ, Bruce J, Zhang T, Kimple RJ, Hong S
2022 Oct 1;213:114445. doi: 10.1016/j.bios.2022.114445. Epub 2022 Jun 1.
-
More
Despite its high potential, PD-L1 expressed by tumors has not been successfully utilized as a biomarker for estimating treatment responses to immunotherapy. Circulating tumor cells (CTCs) and tumor-derived exosomes that express PD-L1 can potentially be used as biomarkers; however, currently available assays lack clinically significant sensitivity and specificity. Here, a novel peptide-based capture surface is developed to effectively isolate PD-L1-expressing CTCs and exosomes from human blood. For the effective targeting of PD-L1, this study integrates peptide engineering strategies to enhance the binding strength and specificity of a β-hairpin peptide derived from PD-1 (pPD-1). Specifically, this study examines the effect of poly(ethylene glycol) spacers, the secondary peptide structure, and modification of peptide sequences (e.g., removal of biologically redundant amino acid residues) on capture efficiency. The optimized pPD-1 configuration captures PD-L1-expressing tumor cells and tumor-derived exosomes with 1.5-fold (p = 0.016) and 1.2-fold (p = 0.037) higher efficiencies, respectively, than their whole antibody counterpart (aPD-L1). This enhanced efficiency is translated into more clinically significant detection of CTCs (1.9-fold increase; p = 0.035) and exosomes (1.5-fold increase; p = 0.047) from patients' baseline samples, demonstrating stronger correlation with patients' treatment responses. Additionally, we confirmed that the clinical accuracy of our system can be further improved by co-analyzing the two biomarkers (bimodal CTC/exosome analysis). These data demonstrate that pPD-1-based capture is a promising approach for capturing PD-L1-expressing CTCs and exosomes, which can be used as a reliable biomarker for cancer immunotherapy.
PMID:35679646 | DOI:10.1016/j.bios.2022.114445
View details for PubMedID 35679646
-
More
-
Stress Keratin 17 Expression in Head and Neck Cancer Contributes to Immune Evasion and Resistance to Immune-Checkpoint Blockade Clinical cancer research : an official journal of the American Association for Cancer Research
Wang W, Lozar T, Golfinos AE, Lee D, Gronski E, Ward-Shaw E, Hayes M, Bruce JY, Kimple RJ, Hu R, Harari PM, Xu J, Keske A, Sondel PM, Fitzpatrick MB, Dinh HQ, Lambert PF
2022 Jul 1;28(13):2953-2968. doi: 10.1158/1078-0432.CCR-21-3039.
-
More
PURPOSE: We investigated whether in human head and neck squamous cell carcinoma (HNSCC) high levels of expression of stress keratin 17 (K17) are associated with poor survival and resistance to immunotherapy.
EXPERIMENTAL DESIGN: We investigated the role of K17 in regulating both the tumor microenvironment and immune responsiveness of HNSCC using a syngeneic mouse HNSCC model, MOC2. MOC2 gives rise to immunologically cold tumors that are resistant to immune-checkpoint blockade (ICB). We engineered multiple, independent K17 knockout (KO) MOC2 cell lines and monitored their growth and response to ICB. We also measured K17 expression in human HNSCC of patients undergoing ICB.
RESULTS: MOC2 tumors were found to express K17 at high levels. When knocked out for K17 (K17KO MOC2), these cells formed tumors that grew slowly or spontaneously regressed and had a high CD8+ T-cell infiltrate in immunocompetent syngeneic C57BL/6 mice compared with parental MOC2 tumors. This phenotype was reversed when we depleted mice for T cells. Whereas parental MOC2 tumors were resistant to ICB treatment, K17KO MOC2 tumors that did not spontaneously regress were eliminated upon ICB treatment. In a cohort of patients with HNSCC receiving pembrolizumab, high K17 expression correlated with poor response. Single-cell RNA-sequencing analysis revealed broad differences in the immune landscape of K17KO MOC2 tumors compared with parental MOC2 tumors, including differences in multiple lymphoid and myeloid cell types.
CONCLUSIONS: We demonstrate that K17 expression in HNSCC contributes to immune evasion and resistance to ICB treatment by broadly altering immune landscapes of tumors.
PMID:35621713 | PMC:PMC9250640 | DOI:10.1158/1078-0432.CCR-21-3039
View details for PubMedID 35621713
-
More
-
Activation of the CREB Coactivator CRTC2 by Aberrant Mitogen Signaling promotes oncogenic functions in HPV16 positive head and neck cancer Neoplasia (New York, N.Y.)
Carper MB, Goel S, Zhang AM, Damrauer JS, Cohen S, Zimmerman MP, Gentile GM, Parag-Sharma K, Murphy RM, Sato K, Nickel KP, Kimple RJ, Yarbrough WG, Amelio AL
2022 Jul;29:100799. doi: 10.1016/j.neo.2022.100799. Epub 2022 Apr 30.
-
More
Head and neck squamous cell carcinoma (HNSCC) is the 6th most common cancer worldwide and incidence rates are continuing to rise globally. Patients often present with locally advanced disease and a staggering 50% chance of relapse following treatment. Aberrant activation of adaptive response signaling pathways, such as the cAMP/PKA pathway, induce an array of genes associated with known cancer pathways that promote tumorigenesis and drug resistance. We identified the cAMP Regulated Transcription Coactivator 2 (CRTC2) to be overexpressed and constitutively activated in HNSCCs and this confers poor prognosis. CRTCs are regulated through their subcellular localization and we show that CRTC2 is exclusively nuclear in HPV(+) HNSCC, thus constitutively active, due to non-canonical Mitogen-Activated Kinase Kinase 1 (MEKK1)-mediated activation via a MEKK1-p38 signaling axis. Loss-of-function and pharmacologic inhibition experiments decreased CRTC2/CREB transcriptional activity by reducing nuclear CRTC2 via nuclear import inhibition and/or by eviction of CRTC2 from the nucleus. This shift in localization was associated with decreased proliferation, migration, and invasion. Our results suggest that small molecules that inhibit nuclear CRTC2 and p38 activity may provide therapeutic benefit to patients with HPV(+) HNSCC.
PMID:35504112 | PMC:PMC9065880 | DOI:10.1016/j.neo.2022.100799
View details for PubMedID 35504112
-
More
-
Using 4D dose accumulation to calculate organ-at-risk dose deviations from motion-synchronized liver and lung tomotherapy treatments Journal of applied clinical medical physics
Ferris WS, Chao EH, Smilowitz JB, Kimple RJ, Bayouth JE, Culberson WS
2022 Jul;23(7):e13627. doi: 10.1002/acm2.13627. Epub 2022 Apr 29.
-
More
Tracking systems such as Radixact Synchrony change the planned delivery of radiation during treatment to follow the target. This is typically achieved without considering the location changes of organs at risk (OARs). The goal of this work was to develop a novel 4D dose accumulation framework to quantify OAR dose deviations due to the motion and tracked treatment. The framework obtains deformation information and the target motion pattern from a four-dimensional computed tomography dataset. The helical tomotherapy treatment plan is split into 10 plans and motion correction is applied separately to the jaw pattern and multi-leaf collimator (MLC) sinogram for each phase based on the location of the target in each phase. Deformable image registration (DIR) is calculated from each phase to the references phase using a commercial algorithm, and doses are accumulated according to the DIR. The effect of motion synchronization on OAR dose was analyzed for five lung and five liver subjects by comparing planned versus synchrony-accumulated dose. The motion was compensated by an average of 1.6 cm of jaw sway and by an average of 5.7% of leaf openings modified, indicating that most of the motion compensation was from jaw sway and not MLC changes. OAR dose deviations as large as 19 Gy were observed, and for all 10 cases, dose deviations greater than 7 Gy were observed. Target dose remained relatively constant (D95% within 3 Gy), confirming that motion-synchronization achieved the goal of maintaining target dose. Dose deviations provided by the framework can be leveraged during the treatment planning process by identifying cases where OAR doses may change significantly from their planned values with respect to the critical constraints. The framework is specific to synchronized helical tomotherapy treatments, but the OAR dose deviations apply to any real-time tracking technique that does not consider location changes of OARs.
PMID:35486094 | PMC:PMC9278681 | DOI:10.1002/acm2.13627
View details for PubMedID 35486094
-
More
-
Prospective Study of PET/MRI Tumor Response During Chemoradiotherapy for Patients With Low-risk and Intermediate-risk p16-positive Oropharynx Cancer American journal of clinical oncology
Witek ME, Kimple RJ, Avey GD, Burr AR, Chandereng T, Yu M, Hu R, Wieland AM, Labby ZE, Bruce JY, Brower JV, Hartig GK, Harari PM
2022 May 1;45(5):202-207. doi: 10.1097/COC.0000000000000910. Epub 2022 Apr 12.
-
More
OBJECTIVE: The objective of this study was to examine tumor response with positron emission tomography (PET)/magnetic resonance imaging (MRI) during chemoradiotherapy as a predictor of outcome in patients with p16-positive oropharynx cancer.
MATERIALS AND METHODS: Patients with p16-positive oropharynx cancer were treated with chemoradiotherapy. Low-risk (LR) disease was defined as T1-T3 and N0-2b and ≤10 pack-years and intermediate-risk (IR) disease as T4 or N2c-3 or >10 pack-years. Patients underwent a PET/MRI scan pretreatment and at fraction 10. Change in value of imaging means were analyzed by analysis of variance. K-means clustering with Euclidean distance functions were used for patient clustering. Silhouette width was used to determine the optimal number of clusters. Linear regression was performed on all radiographic metrics using patient and disease characteristics.
RESULTS: Twenty-four patients were enrolled with 7 LR and 11 IR patients available for analysis. Pretreatment imaging characteristics between LR and IR patients were similar. Patients with LR disease exhibited a larger reduction in maximum standardized uptake value (SUV) compared with IR patients (P<0.05). Cluster analysis defined 2 cohorts that exhibited a similar intratreatment response. Cluster 1 contained 7 of 7 LR patients and 8 of 11 IR patients. Cluster 2 contained 3 of 11 IR patients. Cluster 2 exhibited significant differences compared with cluster 1 in the change in primary tumor peak SUV and largest lymph node median SUV.
CONCLUSIONS: We identified that IR p16-positive oropharynx cancers exhibit heterogeneity in their PET/MRI response to chemoradiotherapy. These data support further study of intratreatment imaging response as a potential mechanism to identify patients with IR oropharynx cancer suitable for treatment deintensification.
PMID:35446279 | PMC:PMC9623610 | DOI:10.1097/COC.0000000000000910
View details for PubMedID 35446279
-
More
-
Advances in Organ Preservation for Laryngeal Cancer Current treatment options in oncology
Campbell G, Glazer TA, Kimple RJ, Bruce JY
2022 Apr;23(4):594-608. doi: 10.1007/s11864-022-00945-5. Epub 2022 Mar 18.
-
More
At the University of Wisconsin, all treatment of head and neck cancer patients begins with discussion at our multi-disciplinary tumor board. Most patients with T4 disease, with existing laryngeal dysfunction, considered unlikely to complete definitive CRT or who have a high risk of persistent aspiration after non-operative management undergo total laryngectomy. A laryngeal sparing approach is attempted on most other patients. Radiotherapy is delivered over 6.5 weeks, preferably with concurrent weekly cisplatin. If the patient is hesitant of chemotherapy or has contraindications to cisplatin, concurrent cetuximab may be offered. Patients treated with RT alone are often treated to the same dose, but via an accelerated schedule by adding a 6th fraction per week. The 6th fraction is given by delivering two treatments at least 6 h apart on a weekday of the patient's choosing. We consider the following to be major risk factors for clinically significant weight loss during treatment: a 10% or greater loss of weight in the 6 months prior to starting treatment, delivery of concurrent cisplatin, and treatment of the bilateral neck with radiation. Patients who have 2-3 of these characteristics are often given gastrostomy tubes prophylactically. Patients are seen 2 weeks after completion of therapy, and then every 3 months after completion for 2 years. A CT neck and PET-CT are performed at the first 3-month visit. They are seen twice in year three, and then yearly until years 5-7. At each of these visits, we have a low threshold to present the patient at our multidisciplinary tumor board for consideration of salvage laryngectomy if there are signs of progression.
PMID:35303749 | PMC:PMC9405127 | DOI:10.1007/s11864-022-00945-5
View details for PubMedID 35303749
-
More
-
Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): Study protocol for a phase 1 dose-escalation trial of patients with xerostomia after radiation therapy for head and neck cancer: MARSH: Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction Cytotherapy
Blitzer GC, Rogus-Pulia NM, Mattison RJ, Varghese T, Ganz O, Chappell R, Galipeau J, McDowell KA, Meyers RO, Glazer TA, Kimple RJ
2022 May;24(5):534-543. doi: 10.1016/j.jcyt.2021.11.003. Epub 2022 Feb 16.
-
More
BACKGROUND: Xerostomia, or dry mouth, is a common side effect of head and neck radiation. Current treatment options for radiation-induced xerostomia are generally supportive in nature. Adult stem cells are the ultimate source for replenishment of salivary gland tissue. Bone marrow-derived mesenchymal stromal cells (BM-MSCs) are a viable cell-based therapy for xerostomia. We have undertaken studies enabling U.S. Food and Drug Administration Investigational New Drug status, demonstrating the normal phenotype, intact functionality, and pro-growth secretome of interferon-γ (IFNγ)-stimulated BM-MSCs taken from patients with head and neck cancer who have undergone radiation ± chemotherapy. Here we present the protocol of MARSH, a first-in-human clinical trial of bone marrow-derived, IFNγ-activated BM-MSCs for the treatment of radiation-induced xerostomia.
METHODS: This single-center phase 1 dose-escalation with expansion cohort, non-placebo-controlled study will assess the safety and tolerability of BM-MSCs for the treatment of radiation-induced xerostomia in patients who had head and neck cancer. The phase 1 dose-escalation study will be a 3 + 3 design with staggered enrollment. A total of 21 to 30 subjects (9 to 18 in phase 1 study, 12 in expansion cohort) will be enrolled. The primary endpoint is determining the recommended phase 2 dose (RP2D) of IFNγ-stimulated BM-MSCs to enable further studies on the efficacy of BM-MSCs. Patients' bone marrow will be aspirated, and BM-MSCs will be expanded, stimulated with IFNγ, and injected into the submandibular gland. The RP2D will be determined by dose-limiting toxicities occurring within 1 month of BM-MSC injection. Secondary outcomes of saliva amounts and composition, ultrasound of salivary glands, and quality of life surveys will be taken at 3-, 6-, 12-, and 24-month visits.
DISCUSSION: Autotransplantation of IFNγ-stimulated BM-MSCs in salivary glands after radiation therapy or chemoradiation therapy may provide an innovative remedy to treat xerostomia and restore quality of life. This is the first therapy for radiation-induced xerostomia that may be curative.
TRIAL REGISTRATION: World Health Organization International Clinical Trials Registry Platform: NCT04489732.
PMID:35183442 | PMC:PMC9038658 | DOI:10.1016/j.jcyt.2021.11.003
View details for PubMedID 35183442
-
More
-
Autophagy awakens-the myriad roles of autophagy in head and neck cancer development and therapeutic response Molecular carcinogenesis
Bradley ST, Lee Y, Gurel Z, Kimple RJ
2022 Feb;61(2):243-253. doi: 10.1002/mc.23372. Epub 2021 Nov 15.
-
More
Autophagy is an evolutionarily conserved cell survival mechanism that degrades damaged proteins and organelles to generate cellular energy during times of stress. Recycling of these cellular components occurs in a series of sequential steps with multiple regulatory points. Mechanistic dysfunction can lead to a variety of human diseases and cancers due to the complexity of autophagy and its ability to regulate vital cellular functions. The role that autophagy plays in both the development and treatment of cancer is highly complex, especially given the fact that most cancer therapies modulate autophagy. This review aims to discuss the balance of autophagy in the development, progression, and treatment of head and neck cancer, as well as highlighting the need for a deeper understanding of what is still unknown about autophagy.
PMID:34780672 | PMC:PMC8799495 | DOI:10.1002/mc.23372
View details for PubMedID 34780672
-
More
-
Defining high-risk elective contralateral neck radiation volumes for oropharynx cancer Head & neck
Witek ME, Woody NM, Musunuru HB, Hill PM, Yadav P, Burr AR, Ko HC, Ross RB, Kimple RJ, Harari PM
2022 Feb;44(2):317-324. doi: 10.1002/hed.26924. Epub 2021 Nov 11.
-
More
BACKGROUND: To define the location of the initial contralateral lymph node (LN) metastasis in patients with oropharynx cancer.
METHODS: The location of the LN centroids from patients with oropharynx cancer and a single radiographically positive contralateral LN was defined. A clinical target volume (CTV) inclusive of all LN centroids was created, and its impact on dose to organs at risk was assessed.
RESULTS: We identified 55 patients of which 49/55 had a single contralateral LN in level IIA, 4/55 in level III, 1/55 in level IIB, and 1/55 in the retropharynx. Mean radiation dose to the contralateral parotid gland was 15.1 and 21.0 Gy, (p <0.001) using the modeled high-risk elective CTV and a consensus CTV, respectively.
CONCLUSIONS: We present a systematic approach for identifying the contralateral nodal regions at highest risk of harboring subclinical disease in patients with oropharynx cancer that warrants prospective clinical study.
PMID:34761832 | PMC:PMC9723806 | DOI:10.1002/hed.26924
View details for PubMedID 34761832
-
More
-
Retraction Science signaling
Brand TM, Iida M, Corrigan KL, Braverman CM, Coan JP, Flanigan BG, Stein AP, Salgia R, Rolff J, Kimple RJ, Wheeler DL
2021 Nov 9;14(708):eabn0168. doi: 10.1126/scisignal.abn0168. Epub 2021 Nov 9.
-
Biology of HPV Mediated Carcinogenesis and Tumor Progression Seminars in radiation oncology
Cosper PF, Bradley S, Luo L, Kimple RJ
2021 Oct;31(4):265-273. doi: 10.1016/j.semradonc.2021.02.006.
-
More
Human papillomavirus (HPV) is a ubiquitous DNA virus that infects squamous epithelia. Though HPV only encodes 8 genes, it is capable of causing cellular transformation and ultimately cancer in host cells. In this article we review the classification of HPV viruses, their genetic structure and life cycle, viral gene biology, and provide an overview of the role of HPV in cancer. We explain how the viral life cycle can lead to integration of viral DNA into the host genome leading to increased cell cycle progression, decreased apoptosis, altered DNA repair, and chromosomal instability. We describe the multifaceted roles of the canonical oncogenes E6 and E7 in promoting tumorigenesis and the important role of other viral genes in regulating cancer development. We also review how the virus actively suppresses innate and adaptive immunity to evade immune detection and promote a pro-tumorigenic microenvironment. The biology presented here will serve as a foundation to the other chapters in this edition and we hope it will incite enthusiasm for continued research on this fascinating virus that causes significant morbidity and mortality worldwide.
PMID:34455982 | PMC:PMC8409095 | DOI:10.1016/j.semradonc.2021.02.006
View details for PubMedID 34455982
-
More
-
ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non-Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts Molecular cancer therapeutics
Baschnagel AM, Elnaggar JH, VanBeek HJ, Kromke AC, Skiba JH, Kaushik S, Abel L, Clark PA, Longhurst CA, Nickel KP, Leal TA, Zhao SG, Kimple RJ
2021 Nov;20(11):2129-2139. doi: 10.1158/1535-7163.MCT-21-0305. Epub 2021 Aug 19.
-
More
M6620, a selective ATP-competitive inhibitor of the ATM and RAD3-related (ATR) kinase, is currently under investigation with radiation in patients with non-small cell lung cancer (NSCLC) brain metastases. We evaluated the DNA damage response (DDR) pathway profile of NSCLC and assessed the radiosensitizing effects of M6620 in a preclinical NSCLC brain metastasis model. Mutation analysis and transcriptome profiling of DDR genes and pathways was performed on NSCLC patient samples. NSCLC cell lines were assessed with proliferation, clonogenic survival, apoptosis, cell cycle, and DNA damage signaling and repair assays. NSCLC brain metastasis patient-derived xenograft models were used to assess intracranial response and overall survival. In vivo IHC was performed to confirm in vitro results. A significant portion of NSCLC patient tumors demonstrated enrichment of DDR pathways. DDR pathways correlated with lung squamous cell histology; and mutations in ATR, ATM, BRCA1, BRCA2, CHEK1, and CHEK2 correlated with enrichment of DDR pathways in lung adenocarcinomas. M6620 reduced colony formation after radiotherapy and resulted in inhibition of DNA DSB repair, abrogation of the radiation-induced G2 cell checkpoint, and formation of dysfunctional micronuclei, leading to enhanced radiation-induced mitotic death. The combination of M6620 and radiation resulted in improved overall survival in mice compared with radiation alone. In vivo IHC revealed inhibition of pChk1 in the radiation plus M6620 group. M6620 enhances the effect of radiation in our preclinical NSCLC brain metastasis models, supporting the ongoing clinical trial (NCT02589522) evaluating M6620 in combination with whole brain irradiation in patients with NSCLC brain metastases.
PMID:34413128 | PMC:PMC8571002 | DOI:10.1158/1535-7163.MCT-21-0305
View details for PubMedID 34413128
-
More
-
Dichotomic Potency of IFNγ Licensed Allogeneic Mesenchymal Stromal Cells in Animal Models of Acute Radiation Syndrome and Graft <em>Versus</em> Host Disease Frontiers in immunology
Chinnadurai R, Bates PD, Kunugi KA, Nickel KP, DeWerd LA, Capitini CM, Galipeau J, Kimple RJ
2021 Jul 26;12:708950. doi: 10.3389/fimmu.2021.708950. eCollection 2021.
-
More
Mesenchymal stromal cells (MSCs) are being tested as a cell therapy in clinical trials for dozens of inflammatory disorders, with varying levels of efficacy reported. Suitable and robust preclinical animal models for testing the safety and efficacy of different types of MSC products before use in clinical trials are rare. We here introduce two highly robust animal models of immune pathology: 1) acute radiation syndrome (ARS) and 2) graft versus host disease (GvHD), in conjunction with studying the immunomodulatory effect of well-characterized Interferon gamma (IFNγ) primed bone marrow derived MSCs. The animal model of ARS is based on clinical grade dosimetry precision and bioluminescence imaging. We found that allogeneic MSCs exhibit lower persistence in naïve compared to irradiated animals, and that intraperitoneal infusion of IFNγ prelicensed allogeneic MSCs protected animals from radiation induced lethality by day 30. In direct comparison, we also investigated the effect of IFNγ prelicensed allogeneic MSCs in modulating acute GvHD in an animal model of MHC major mismatched bone marrow transplantation. Infusion of IFNγ prelicensed allogeneic MSCs failed to mitigate acute GvHD. Altogether our results demonstrate that infused IFNγ prelicensed allogeneic MSCs protect against lethality from ARS, but not GvHD, thus providing important insights on the dichotomy of IFNγ prelicensed allogenic MSCs in well characterized and robust animal models of acute tissue injury.
PMID:34386012 | PMC:PMC8352793 | DOI:10.3389/fimmu.2021.708950
View details for PubMedID 34386012
-
More
-
RTAnswers Online Patient Education Materials Deviate From Recommended Reading Levels Applied radiation oncology
Rosenberg SA, Denu RA, Francis D, Hullett CR, Fisher M, Schuster JM, Bassetti MF, Kimple RJ
2018;7(2):26-30. Epub 2018 Jun 19.
-
More
OBJECTIVE: Patients are turning to the Internet more often for cancer-related information. Oncology organizations need to ensure that appropriately written information is available for patients online. The aim of this study was to determine whether the readability of radiation oncology online patient information (OPI) provided by RTAnswers (RTAnswers.org, created by the American Society for Radiation Oncology) is written at a sixth-grade level as recommended by the National Institutes of Health (NIH), the U.S Department of Health and Human Services (HHS), and the American Medical Association (AMA).
METHODS: RTanswers.org was accessed and online patient-oriented brochures for 13 specific disease sites were analyzed. Readability of OPI from RTAnswers was assessed using 10 common readability tests: New Dale-Chall Test, Flesch Reading Ease Score, Coleman-Liau Index, Flesch-Kinkaid Grade Level, FORCAST test, Fry Score, Simple Measure of Gobbledygook, Gunning Frequency of Gobbledygook, New Fog Count, and Raygor Readability Estimate.
RESULTS: A composite grade level of readability was constructed using the 8 readability measures that provide a single grade-level output. The grade levels computed by each of these 8 tests were highly correlated (SI alpha = 0.98). The composite grade level for these disease site-specific brochures was 11.6 ± 0.83, corresponding to a senior in high school, significantly higher than the target sixth-grade level (p < 0.05) recommended by the NIH, HHS, and AMA.
CONCLUSION: Patient educational material provided by RTAnswers.org is written significantly above the target reading level. Simplifying and rewording this information could improve patients' understanding of radiation therapy and improve treatment adherence and outcomes.
PMID:34169120 | PMC:PMC8221236
View details for PubMedID 34169120
-
More
-
Recommendations for postoperative radiotherapy in head & neck squamous cell carcinoma in the presence of flaps: A GORTEC internationally-reviewed HNCIG-endorsed consensus Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Carsuzaa F, Lapeyre M, Gregoire V, Maingon P, Beddok A, Marcy P, Salleron J, Coutte A, Racadot S, Pointreau Y, Graff P, Beadle B, Benezery K, Biau J, Calugaru V, Castelli J, Chua M, Rito AD, Dore M, Ghadjar P, Huguet F, Jardel P, Johansen J, Kimple R, Krengli M, Laskar S, Mcdowell L, Nichols A, Tribius S, Valduvieco I, Hu C, Liem X, Moya-Plana A, D'onofrio I, Parvathaneni U, Takiar V, Orlandi E, Psyrri A, Shenouda G, Sher D, Steuer C, Sun XS, Tao Y, Thomson D, Tsai M, Vulquin N, Gorphe P, Mehanna H, Yom SS, Bourhis J, Thariat J
2021 Jul;160:140-147. doi: 10.1016/j.radonc.2021.04.026. Epub 2021 May 11.
-
More
INTRODUCTION: Head and neck reconstructive surgery using a flap is increasingly common. Best practices and outcomes for postoperative radiotherapy (poRT) with flaps have not been specified. We aimed to provide consensus recommendations to assist clinical decision-making highlighting areas of uncertainty in the presence of flaps.
MATERIAL AND METHODS: Radiation, medical, and surgical oncologists were assembled from GORTEC and internationally with the Head and Neck Cancer International Group (HNCIG). The consensus-building approach covered 59 topics across four domains: (1) identification of postoperative tissue changes on imaging for flap delineation, (2) understanding of tumor relapse risks and target volume definitions, (3) functional radiation-induced deterioration, (4) feasibility of flap avoidance.
RESULTS: Across the 4 domains, international consensus (median score ≥ 7/9) was achieved only for functional deterioration (73.3%); other consensus rates were 55.6% for poRT avoidance of flap structures, 41.2% for flap definition and 11.1% for tumor spread patterns. Radiation-induced flap fibrosis or atrophy and their functional impact was well recognized while flap necrosis was not, suggesting dose-volume adaptation for the former. Flap avoidance was recommended to minimize bone flap osteoradionecrosis but not soft-tissue toxicity. The need for identification (CT planning, fiducials, accurate operative report) and targeting of the junction area at risk between native tissues and flap was well recognized. Experts variably considered flaps as prone to tumor dissemination or not. Discrepancies in rating of 11 items among international reviewing participants are shown.
CONCLUSION: International GORTEC and HNCIG-endorsed recommendations were generated for the management of flaps in head and neck radiotherapy. Considerable knowledge gaps hinder further consensus, in particular with respect to tumor spread patterns.
PMID:33984351 | DOI:10.1016/j.radonc.2021.04.026
View details for PubMedID 33984351
-
More
-
Impact of immediate cryopreservation on the establishment of patient derived xenografts from head and neck cancer patients Journal of translational medicine
Abel L, Durmaz A, Hu R, Longhurst C, Baschnagel AM, Wheeler D, Scott JG, Kimple RJ
2021 Apr 28;19(1):180. doi: 10.1186/s12967-021-02850-1.
-
More
BACKGROUND: Patient-derived xenografts established from human cancers are important tools for investigating novel anti-cancer therapies. Establishing PDXs requires a significant investment and many PDXs may be used infrequently due to their similarity to existing models, their growth rate, or the lack of relevant mutations. We performed this study to determine whether we could efficiently establish PDXs after cryopreservation to allow molecular profiling to be completed prior to implanting the human cancer.
METHODS: Fresh tumor was split with half used to establish a PDX immediately and half cryopreserved for later implantation. Resulting tumors were assessed histologically and tumors established from fresh or cryopreserved tissues compared as to the growth rate, extent of tumor necrosis, mitotic activity, keratinization, and grade. All PDXs were subjected to short tandem repeat testing to confirm identity and assess similarity between methods.
RESULTS: Tumor growth was seen in 70% of implanted cases. No growth in either condition was seen in 30% of tumors. One developed a SCC from the immediate implant but a lymphoproliferative mass without SCC from the cryopreserved specimen. No difference in growth rate was seen. No difference between histologic parameters was seen between the two approaches.
CONCLUSIONS: Fresh human cancer tissue can be immediately cryopreserved and later thawed and implanted to establish PDXs. This resource saving approach allows for tumor profiling prior to implantation into animals thus maximizing the probability that the tumor will be utilized for future research.
PMID:33910584 | PMC:PMC8082827 | DOI:10.1186/s12967-021-02850-1
View details for PubMedID 33910584
-
More
-
Clinical Cancer Advances 2021: ASCO's Report on Progress Against Cancer Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Smith SM, Wachter K, Burris HA, Schilsky RL, George DJ, Peterson DE, Johnson ML, Markham MJ, Mileham KF, Beg MS, Bendell JC, Dreicer R, Keedy VL, Kimple RJ, Knoll MA, LoConte N, MacKay H, Meisel JL, Moynihan TJ, Mulrooney DA, Mulvey TM, Odenike O, Pennell NA, Reeder-Hayes K, Smith C, Sullivan RJ, Uzzo R
2021 Apr 1;39(10):1165-1184. doi: 10.1200/JCO.20.03420. Epub 2021 Feb 2.
-
Immunotherapy in Head and Neck Cancer-Ready for Prime Time or More Research Needed? International journal of radiation oncology, biology, physics
Karam SD, Anderson CM, Ma D, Chua LK, Kimple RJ
2021 Mar 1;109(3):647-650. doi: 10.1016/j.ijrobp.2020.11.022.
-
More
PMID:33516431 | PMC:PMC8597385 | DOI:10.1016/j.ijrobp.2020.11.022
View details for PubMedID 33516431
-
More
-
Development and characterization of patient-derived xenografts from non-small cell lung cancer brain metastases Scientific reports
Baschnagel AM, Kaushik S, Durmaz A, Goldstein S, Ong IM, Abel L, Clark PA, Gurel Z, Leal T, Buehler D, Iyer G, Scott JG, Kimple RJ
2021 Jan 28;11(1):2520. doi: 10.1038/s41598-021-81832-1.
-
More
Non-small cell lung cancer (NSCLC) brain metastasis cell lines and in vivo models are not widely accessible. Herein we report on a direct-from patient-derived xenograft (PDX) model system of NSCLC brain metastases with genomic annotation useful for translational and mechanistic studies. Both heterotopic and orthotopic intracranial xenografts were established and RNA and DNA sequencing was performed on patient and matching tumors. Morphologically, strong retention of cytoarchitectural features was observed between original patient tumors and PDXs. Transcriptome and mutation analysis revealed high correlation between matched patient and PDX samples with more than more than 95% of variants detected being retained in the matched PDXs. PDXs demonstrated response to radiation, response to selumetinib in tumors harboring KRAS G12C mutations and response to savolitinib in a tumor with MET exon 14 skipping mutation. Savolitinib also demonstrated in vivo radiation enhancement in our MET exon 14 mutated PDX. Early passage cell strains showed high consistency between patient and PDX tumors. Together, these data describe a robust human xenograft model system for investigating NSCLC brain metastases. These PDXs and cell lines show strong phenotypic and molecular correlation with the original patient tumors and provide a valuable resource for testing preclinical therapeutics.
PMID:33510214 | PMC:PMC7843608 | DOI:10.1038/s41598-021-81832-1
View details for PubMedID 33510214
-
More
-
Mentorship Initiatives in Radiation Oncology: A Scoping Review of the Literature International journal of radiation oncology, biology, physics
Marsiglio JA, Rosenberg DM, Rooney MK, Goodman CR, Gillespie EF, Hirsch AE, Holliday EB, Kimple RJ, Thomas CR, Golden DW
2021 Jun 1;110(2):292-302. doi: 10.1016/j.ijrobp.2020.12.049. Epub 2021 Jan 4.
-
More
PURPOSE: Although mentorship is described extensively in academic medical literature, there are few descriptions of mentorship specific to radiation oncology. The goal of the current study was to investigate the state of mentorship in radiation oncology through a scoping review of the literature.
METHODS AND MATERIALS: A search protocol was defined according to Preferred Reporting Items for Systematic Reviews and Meta Analyses extension for scoping reviews (PRISMA-ScR) guidelines. Predefined search terms and medical subject headings were used to search PubMed for English language articles published after January 1, 1990, on mentorship in radiation oncology. Additionally, in-press articles from major radiation oncology and medical education journals were searched. Three reviewers determined article eligibility. Included articles were classified based on predefined evaluation criteria.
RESULTS: Fourteen publications from 2008 to 2019 met inclusion criteria. The most commonly described form of mentorship was the dyad (64.3%), followed by team (14.3%) and peer (7.1%); 2 articles did not specify mentorship type (14.3%). The most commonly mentored participants were residents (35.7%), followed by medical students (35.7%) and attendings (21.4%); 1 study included participants of all levels (7.1%). Thirteen studies (92.9%) identified an experimental study design, most of which were cross-sectional (42.9%), followed by cohort studies (28.6%) and before/after (21.4%). Median sample size, reported in 12 of 13 experimental studies, was 132 (coefficient of variation, 1.06). Although outcomes varied widely, the majority described successful implementation of mentorship initiatives with high levels of participant satisfaction.
CONCLUSIONS: Although few initiatives are currently reported, the present study suggests that these initiatives are successful in promoting career development and increasing professional satisfaction. The interventions overwhelmingly described mentorship dyads; other forms of mentorship are either less common or understudied. Limitations included interventions not being evaluated in a controlled setting, and many were assessed using surveys with low response rates. This review highlights rich opportunities for future scholarship to develop, evaluate, and disseminate radiation oncology mentorship initiatives.
PMID:33412265 | PMC:PMC8122025 | DOI:10.1016/j.ijrobp.2020.12.049
View details for PubMedID 33412265
-
More
-
AXL Mediates Cetuximab and Radiation Resistance Through Tyrosine 821 and the c-ABL Kinase Pathway in Head and Neck Cancer Clinical cancer research : an official journal of the American Association for Cancer Research
McDaniel NK, Iida M, Nickel KP, Longhurst CA, Fischbach SR, Rodems TS, Kranjac CA, Bo AY, Luo Q, Gallagher MM, Welke NB, Mitchell KR, Schulz AE, Eckers JC, Hu R, Salgia R, Hong S, Bruce JY, Kimple RJ, Wheeler DL
2020 Aug 15;26(16):4349-4359. doi: 10.1158/1078-0432.CCR-19-3142. Epub 2020 May 21.
-
More
PURPOSE: Radiation and cetuximab are therapeutics used in management of head and neck squamous cell carcinoma (HNSCC). Despite clinical success with these modalities, development of both intrinsic and acquired resistance is an emerging problem in the management of this disease. The purpose of this study was to investigate signaling of the receptor tyrosine kinase AXL in resistance to radiation and cetuximab treatment.
EXPERIMENTAL DESIGN: To study AXL signaling in the context of treatment-resistant HNSCC, we used patient-derived xenografts (PDXs) implanted into mice and evaluated the tumor response to AXL inhibition in combination with cetuximab or radiation treatment. To identify molecular mechanisms of how AXL signaling leads to resistance, three tyrosine residues of AXL (Y779, Y821, Y866) were mutated and examined for their sensitivity to cetuximab and/or radiation. Furthermore, reverse phase protein array (RPPA) was employed to analyze the proteomic architecture of signaling pathways in these genetically altered cell lines.
RESULTS: Treatment of cetuximab- and radiation-resistant PDXs with AXL inhibitor R428 was sufficient to overcome resistance. RPPA analysis revealed that such resistance emanates from signaling of tyrosine 821 of AXL via the tyrosine kinase c-ABL. In addition, inhibition of c-ABL signaling resensitized cells and tumors to cetuximab or radiotherapy even leading to complete tumor regression without recurrence in head and neck cancer models.
CONCLUSIONS: Collectively, the studies presented herein suggest that tyrosine 821 of AXL mediates resistance to cetuximab by activation of c-ABL kinase in HNSCC and that targeting of both EGFR and c-ABL leads to a robust antitumor response.
PMID:32439698 | PMC:PMC7442604 | DOI:10.1158/1078-0432.CCR-19-3142
View details for PubMedID 32439698
-
More
-
Follow-Up and Management of Patients With Head and Neck Cancer During the 2019 Novel Coronavirus (SARS-CoV-2) Disease Pandemic Advances in radiation oncology
Chua LK, Ma DJ, Anderson CM, Karam SD, Margalit DN, Kimple RJ
2020 May 15;5(4):631-636. doi: 10.1016/j.adro.2020.04.031. eCollection 2020 Jul-Aug.
-
Immunoavidity-Based Capture of Tumor Exosomes Using Poly(amidoamine) Dendrimer Surfaces Nano letters
Poellmann MJ, Nair A, Bu J, Kim KH, Kimple RJ, Hong S
2020 Aug 12;20(8):5686-5692. doi: 10.1021/acs.nanolett.0c00950. Epub 2020 May 19.
-
More
Tumor-derived blood-circulating exosomes have potential as a biomarker to greatly improve cancer treatment. However, effective isolation of exosomes remains a tremendous technical challenge. This study presents a novel nanostructured polymer surface for highly effective capture of exosomes through strong avidity. Various surface configurations, consisting of multivalent dendrimers, PEG, and tumor-targeting antibodies, were tested using exosomes isolated from tumor cell lines. We found that a dual layer dendrimer configuration exhibited the highest efficiency in capturing cultured exosomes spiked into human serum. Importantly, the optimized surface captured a > 4-fold greater amount of tumor exosomes from head and neck cancer patient plasma samples than that from healthy donors. Nanomechanical analysis using atomic force microscopy also revealed that the enhancement was attributed to multivalent binding (avidity) and augmented short-range adhesion mediated by dendrimers. Our results support that the dendrimer surface detects tumor exosomes at high sensitivity and specificity, demonstrating its potential as a new cancer liquid biopsy platform.
PMID:32407121 | PMC:PMC12180921 | DOI:10.1021/acs.nanolett.0c00950
View details for PubMedID 32407121
-
More
-
FGFR Inhibition Enhances Sensitivity to Radiation in Non-Small Cell Lung Cancer Molecular cancer therapeutics
SenthilKumar G, Fisher MM, Skiba JH, Miller MC, Brennan SR, Kaushik S, Bradley ST, Longhurst CA, Buehler D, Nickel KP, Iyer G, Kimple RJ, Baschnagel AM
2020 Jun;19(6):1255-1265. doi: 10.1158/1535-7163.MCT-19-0931. Epub 2020 May 5.
-
More
FGFRs are commonly altered in non-small cell lung cancer (NSCLC). FGFRs activate multiple pathways including RAS/RAF/MAPK, PI3K/AKT, and STAT, which may play a role in the cellular response to radiation. We investigated the effects of combining the selective FGFR 1-3 tyrosine kinase inhibitor AZD4547 with radiation in cell line and xenograft models of NSCLC. NSCLC cell lines were assessed with proliferation, clonogenic survival, apoptosis, autophagy, cell cycle, and DNA damage signaling and repair assays. In vivo xenografts and IHC were used to confirm in vitro results. NSCLC cell lines demonstrated varying degrees of FGFR protein and mRNA expression. In vitro clonogenic survival assays showed radiosensitization with AZD4547 in two NSCLC cell lines. In these two cell lines, an increase in apoptosis and autophagy was observed with combined radiation and AZD4547. The addition of AZD4547 to radiation did not significantly affect γH2AX foci formation. Enhanced xenograft tumor growth delay was observed with the combination of radiation and AZD4547 compared with radiation or drug alone. IHC results revealed inhibition of pMAPK and pS6 and demonstrated an increase in apoptosis in the radiation plus AZD4547 group. This study demonstrates that FGFR inhibition by AZD4547 enhances the response of radiation in FGFR-expressing NSCLC in vitro and in vivo model systems. These results support further investigation of combining FGFR inhibition with radiation as a clinical therapeutic strategy.
PMID:32371583 | PMC:PMC7272291 | DOI:10.1158/1535-7163.MCT-19-0931
View details for PubMedID 32371583
-
More
-
Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas International journal of radiation oncology, biology, physics
Fisher MM, SenthilKumar G, Hu R, Goldstein S, Ong IM, Miller MC, Brennan SR, Kaushik S, Abel L, Nickel KP, Iyer G, Harari PM, Kimple RJ, Baschnagel AM
2020 Jul 15;107(4):793-803. doi: 10.1016/j.ijrobp.2020.03.040. Epub 2020 Apr 13.
-
More
PURPOSE: We examined the capacity of the pan-fibroblast growth factor receptor (FGFR) inhibitor AZD4547 to augment radiation response across a panel of head and neck squamous cell carcinoma (HNSCC) cell lines and xenografts.
METHODS AND MATERIALS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization expression was assessed in a cohort of HNSCC patient samples, cell lines, and patient-derived xenografts (PDXs). In vitro effects of AZD4547 and radiation on cell survival, FGFR signaling, apoptosis, autophagy, cell cycle, and DNA damage repair were evaluated. Reverse phase protein array was used to identify differentially phosphorylated proteins in cells treated with AZD4547. In vivo tumor responses were evaluated in cell lines and PDX models.
RESULTS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization were expressed in 41%, 81%, and 89% of 107 oropharynx patient samples. Sensitivity to AZD4547 did not directly correlate with FGFR protein or RNA expression. In sensitive cell lines, AZD4547 inhibited p-MAPK in a time-dependent manner. Significant radiosensitization with AZD4547 was observed in cell lines that were sensitive to AZD4547. The mechanism underlying these effects appears to be multifactorial, involving inhibition of the MTOR pathway and subsequent enhancement of autophagy and activation of apoptotic pathways. Significant tumor growth delay was observed when AZD4547 was combined with radiation compared with radiation or drug alone in an FGFR-expressing HNSCC cell line xenograft and PDX.
CONCLUSIONS: These findings suggest that AZD4547 can augment the response of radiation in FGFR-expressing HNSCC in vivo model systems. FGFR1 and FGFR2 may prove worthy targets for radiosensitization in HNSCC clinical investigations.
PMID:32298810 | PMC:PMC7321889 | DOI:10.1016/j.ijrobp.2020.03.040
View details for PubMedID 32298810
-
More
-
Four Influential Clinical Trials in Human Papilloma Virus-Associated Oropharynx Cancer International journal of radiation oncology, biology, physics
Margalit DN, Karam SD, Chua LK, Anderson C, Kimple RJ
2020 Apr 1;106(5):893-899. doi: 10.1016/j.ijrobp.2019.12.015.
-
Interstitial diffuse optical probe with spectral fitting to measure dynamic tumor hypoxia Biomedical physics & engineering express
Fru LC, Jacques SL, Nickel KP, Varghese T, Kissick MW, DeWerd LA, Kimple RJ
2020 Jan;6(1):10.1088/2057-1976/ab6e16. doi: 10.1088/2057-1976/ab6e16. Epub 2020 Jan 31.
-
More
Understanding the dynamic nature of tumor hypoxia is vital for cancer therapy. The presence of oxygen within a tumor during radiation therapy increases the likelihood of local control. We used a novel interstitial diffuse optical probe to make real-time measurements of blood volume fraction and hemoglobin oxygen saturation within a tumor at a high temporal resolution. This device was initially characterized and benchmarked using a customized vessel designed to control hemoglobin oxygen saturation and blood volume in a solution of blood with different concentrations of an oxygen scavenger, tetrakis (hydroxymethyl) phosphonium chloride. The optical device was found to consistently monitor the changes in oxygen saturation and these changes correlated to the concentration of the oxygen scavenger added. In near-simultaneous measurements of blood volume and oxygen saturation in tumor-bearing mice, the changes in blood volume fraction and oxygen saturation measured with the interstitial diffuse optical probe were benchmarked against photoacoustic imaging system to track and compare temporal dynamics of oxygen saturation and blood volume in a patient-derived xenograft model of hypopharyngeal carcinoma. Positive correlations between our device and photoacoustic imaging in measuring blood volume and oxygen saturation were observed.
PMID:32095273 | PMC:PMC7039661 | DOI:10.1088/2057-1976/ab6e16
View details for PubMedID 32095273
-
More
-
Patient Derived Models to Study Head and Neck Cancer Radiation Response Cancers
Cosper PF, Abel L, Lee Y, Paz C, Kaushik S, Nickel KP, Alexandridis R, Scott JG, Bruce JY, Kimple RJ
2020 Feb 12;12(2):419. doi: 10.3390/cancers12020419.
-
More
Patient-derived model systems are important tools for studying novel anti-cancer therapies. Patient-derived xenografts (PDXs) have gained favor over the last 10 years as newer mouse strains have improved the success rate of establishing PDXs from patient biopsies. PDXs can be engrafted from head and neck cancer (HNC) samples across a wide range of cancer stages, retain the genetic features of their human source, and can be treated with both chemotherapy and radiation, allowing for clinically relevant studies. Not only do PDXs allow for the study of patient tissues in an in vivo model, they can also provide a renewable source of cancer cells for organoid cultures. Herein, we review the uses of HNC patient-derived models for radiation research, including approaches to establishing both orthotopic and heterotopic PDXs, approaches and potential pitfalls to delivering chemotherapy and radiation to these animal models, biological advantages and limitations, and alternatives to animal studies that still use patient-derived tissues.
PMID:32059418 | PMC:PMC7072508 | DOI:10.3390/cancers12020419
View details for PubMedID 32059418
-
More
-
Clinical outcomes for larynx patients with cancer treated with refinement of high-dose radiation treatment volumes Head & neck
Burr AR, Harari PM, Haasl AM, Wieland AM, Bruce JY, Kimple RJ, Hartig GK, McCulloch TM, Witek ME
2020 Aug;42(8):1874-1881. doi: 10.1002/hed.26098. Epub 2020 Feb 14.
-
More
BACKGROUND: To evaluate disease control, toxicities, and dose to dysphagia/aspiration risk structures (DARS) using a direct gross tumor volume (GTV70Gy ) to planning target volume expansion (dPTV70Gy ) for patients with squamous cell carcinoma of the larynx (LSCC).
METHODS: A retrospective review was performed on patients with LSCC treated between 2003 and 2018. Clinical outcomes, toxicities, and dosimetric data were analyzed.
RESULTS: Seventy-three patients were identified. Overall survival at 5-years was 57.8%. Five-year local and regional control was 79.8% and 88.2%, respectively. Distant metastatic-only failure was 2.7%. Eighty percent of failures were 95% contained within the dPTV70Gy . Mean dose and the volume of DARS receiving 70 Gy was significantly lower for dPTV70Gy compared to a consensus-defined PTV70Gy .
DISCUSSION: Judicious reduction in high-dose target volumes can preserve high tumor control rates while reducing dose to normal surrounding structures underscoring the potential benefit of this approach in enabling local therapy intensification to improve locoregional control.
PMID:32057151 | PMC:PMC7369226 | DOI:10.1002/hed.26098
View details for PubMedID 32057151
-
More
-
Clinical Cancer Advances 2020: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, Diefenbach SM, Rodriguez-Galindo C, George DJ, Gilligan TD, Harvey RD, Johnson ML, Kimple RJ, Knoll MA, LoConte N, Maki RG, Meisel JL, Meyerhardt JA, Pennell NA, Rocque GB, Sabel MS, Schilsky RL, Schneider BJ, Tap WD, Uzzo RG, Westin SN
2020 Apr 1;38(10):1081. doi: 10.1200/JCO.19.03141. Epub 2020 Feb 4.
-
More
Shortly before I was elected President of ASCO, I attended the 65th birthday party of a current patient. She had been diagnosed 10 years earlier with metastatic breast cancer and hadn't been sure she wanted to move forward with further treatment. With encouragement, she elected to participate in a clinical trial of an investigational drug that is now widely used to treat breast cancer. Happily, here we were, celebrating with her now-married daughters, their husbands, and three beautiful grandchildren, ages 2, 4, and 8. Such is the importance of clinical trials and promising new therapies.Clinical research is about saving and improving the lives of individuals with cancer. It's a continuing story that builds on the efforts of untold numbers of researchers, clinicians, caregivers, and patients. ASCO's Clinical Cancer Advances report tells part of this story, sharing the most transformative research of the past year. The report also includes our latest thinking on the most urgent research priorities in oncology.ASCO's 2020 Advance of the Year-Refinement of Surgical Treatment of Cancer-highlights how progress drives more progress. Surgery has played a fundamental role in cancer treatment. It was the only treatment available for many cancers until the advent of radiation and chemotherapy. The explosion in systemic therapies since then has resulted in significant changes to when and how surgery is performed to treat cancer. In this report, we explore how treatment successes have led to less invasive approaches for advanced melanoma, reduced the need for surgery in renal cell carcinoma, and increased the number of patients with pancreatic cancer who can undergo surgery.Many research advances are made possible by federal funding. With the number of new US cancer cases set to rise by roughly a third over the next decade, continued investment in research at the national level is crucial to continuing critical progress in the prevention, screening, diagnosis, and treatment of cancer.While clinical research has translated to longer survival and better quality of life for many patients with cancer, we can't rest on our laurels. With ASCO's Research Priorities to Accelerate Progress Against Cancer, introduced last year and updated this year, we've identified the critical gaps in cancer prevention and care that we believe to be most pressing. These priorities are intended to guide the direction of research and speed progress.Of course, the effectiveness or number of new treatments is meaningless if patients don't have access to them. High-quality cancer care, including clinical trials, is out of reach for too many patients. Creating an infrastructure to support patients is a critical part of the equation, as is creating connections between clinical practices and research programs. We have much work to do before everyone with cancer has equal access to the best treatments and the opportunity to participate in research. I know that ASCO and the cancer community are up for this challenge.Sincerely,Howard A. "Skip" Burris III, MD, FACP, FASCOASCO President, 2019-2020.
PMID:32013670 | DOI:10.1200/JCO.19.03141
View details for PubMedID 32013670
-
More
-
STAT-ART: The Promise and Practice of a Rapid Palliative Single Session of MR-Guided Online Adaptive Radiotherapy (ART) Frontiers in oncology
Mittauer KE, Hill PM, Geurts MW, Costa AD, Kimple RJ, Bassetti MF, Bayouth JE
2019 Oct 22;9:1013. doi: 10.3389/fonc.2019.01013. eCollection 2019.
-
More
This work describes a novel application of MR-guided online adaptive radiotherapy (MRgoART) in the management of patients whom urgent palliative care is indicated using statum-adaptive radiotherapy (STAT-ART). The implementation of STAT-ART, as performed at our institution, is presented including a discussion of the advantages and limitations compared to the standard of care for palliative radiotherapy on conventional c-arm linacs. MR-based treatment planning techniques of STAT-ART for density overrides and deformable image registration (DIR) of diagnostic CT to the treatment MR are also addressed.
PMID:31696053 | PMC:PMC6817496 | DOI:10.3389/fonc.2019.01013
View details for PubMedID 31696053
-
More
-
Effects of culture method on response to EGFR therapy in head and neck squamous cell carcinoma cells Scientific reports
Ayuso JM, Vitek R, Swick AD, Skala MC, Wisinski KB, Kimple RJ, Lambert PF, Beebe DJ
2019 Aug 28;9(1):12480. doi: 10.1038/s41598-019-48764-3.
-
More
The EGFR pathway plays a critical role in head and neck squamous cell carcinoma (HNSCC). Targeted therapies against the EGFR are utilized as a treatment for HNSCCC. However, patient response is heterogeneous and molecular biomarkers are lacking to predict patient response. Therefore, functional assays where drug response is directly evaluated in tumor cells are an interesting alternative. Previous studies have shown that experimental conditions modify the drug response observed in functional assays. Thus, in this work the influence of the culture environment on response to Cetuximab (EGFR monoclonal antibody) and AZD8055 (mTOR inhibitor) was evaluated. HNSCC UM-SCC-1 and UM-SCC-47 cells were cultured in 2D monoculture and compared with: 2D co-culture with cancer-associated fibroblasts (CAF); 3D culture in collagen hydrogels; and 3D culture in tumor spheroids. The results showed UM-SCC-1 drug response significantly changed in the different culture environments; leading to an increase in drug resistance in the CAF co-culture and the 3D spheroids. Conversely, UM-SCC-47 exhibited a more constant drug response across culture conditions. In conclusion, this work highlights the importance of culture conditions that modulate response to EGFR pathway inhibition.
PMID:31462653 | PMC:PMC6713778 | DOI:10.1038/s41598-019-48764-3
View details for PubMedID 31462653
-
More
-
High-throughput quantitative detection of basal autophagy and autophagic flux using image cytometry BioTechniques
SenthilKumar G, Skiba JH, Kimple RJ
2019 Aug;67(2):70-73. doi: 10.2144/btn-2019-0044. Epub 2019 Jun 26.
-
More
Quantitative assessment of changes in macro-autophagy is often performed through manual quantification of the number of LC3B foci in immunofluorescence microscopy images. This method is highly laborious, subject to image-field selection and foci-counting bias, and is not sensitive for analyzing changes in basal autophagy. Alternative methods such as flow cytometry and transmission electron microscopy require highly specialized, expensive instruments and time-consuming sample preparation. Immunoblots are prone to exposure-related variations and noise that prevents accurate quantification. We report a high-throughput, inexpensive, reliable and objective method for studying basal level and flux changes in late-stage autophagy using image cytometry and acridine orange staining.
PMID:31238709 | PMC:PMC7141596 | DOI:10.2144/btn-2019-0044
View details for PubMedID 31238709
-
More
-
De-Escalation Strategies in HPV-Associated Oropharynx Cancer-Are we Putting the Cart Before the Horse? International journal of radiation oncology, biology, physics
Anderson CM, Kimple RJ, Lin A, Karam SD, Margalit DN, Chua LK
2019 Jul 15;104(4):705-709. doi: 10.1016/j.ijrobp.2019.02.054.
-
More
PMID:31204653 | PMC:PMC7194352 | DOI:10.1016/j.ijrobp.2019.02.054
View details for PubMedID 31204653
-
More
-
Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation Clinical cancer research : an official journal of the American Association for Cancer Research
Pasch CA, Favreau PF, Yueh AE, Babiarz CP, Gillette AA, Sharick JT, Karim MR, Nickel KP, DeZeeuw AK, Sprackling CM, Emmerich PB, DeStefanis RA, Pitera RT, Payne SN, Korkos DP, Clipson L, Walsh CM, Miller D, Carchman EH, Burkard ME, Lemmon KK, Matkowskyj KA, Newton MA, Ong IM, Bassetti MF, Kimple RJ, Skala MC, Deming DA
2019 Sep 1;25(17):5376-5387. doi: 10.1158/1078-0432.CCR-18-3590. Epub 2019 Jun 7.
-
More
PURPOSE: Cancer treatment is limited by inaccurate predictors of patient-specific therapeutic response. Therefore, some patients are exposed to unnecessary side effects and delays in starting effective therapy. A clinical tool that predicts treatment sensitivity for individual patients is needed.
EXPERIMENTAL DESIGN: Patient-derived cancer organoids were derived across multiple histologies. The histologic characteristics, mutation profile, clonal structure, and response to chemotherapy and radiation were assessed using bright-field and optical metabolic imaging on spheroid and single-cell levels, respectively.
RESULTS: We demonstrate that patient-derived cancer organoids represent the cancers from which they were derived, including key histologic and molecular features. These cultures were generated from numerous cancers, various biopsy sample types, and in different clinical settings. Next-generation sequencing reveals the presence of subclonal populations within the organoid cultures. These cultures allow for the detection of clonal heterogeneity with a greater sensitivity than bulk tumor sequencing. Optical metabolic imaging of these organoids provides cell-level quantification of treatment response and tumor heterogeneity allowing for resolution of therapeutic differences between patient samples. Using this technology, we prospectively predict treatment response for a patient with metastatic colorectal cancer.
CONCLUSIONS: These studies add to the literature demonstrating feasibility to grow clinical patient-derived organotypic cultures for treatment effectiveness testing. Together, these culture methods and response assessment techniques hold great promise to predict treatment sensitivity for patients with cancer undergoing chemotherapy and/or radiation.
PMID:31175091 | PMC:PMC6726566 | DOI:10.1158/1078-0432.CCR-18-3590
View details for PubMedID 31175091
-
More
-
Reducing radiotherapy target volume expansion for patients with HPV-associated oropharyngeal cancer Oral oncology
Burr AR, Harari PM, Ko HC, Bruce JY, Kimple RJ, Witek ME
2019 May;92:52-56. doi: 10.1016/j.oraloncology.2019.03.013. Epub 2019 Mar 22.
-
More
PURPOSE: To evaluate clinical outcomes and patterns of failure using a direct gross tumor volume to planning target volume expansion in patients with p16-positive oropharyngeal squamous cell carcinoma.
METHODS AND MATERIALS: We performed a retrospective review of patients with p16-positive oropharyngeal squamous cell carcinomas treated between 2002 and 2017 with primary radiotherapy with or without concurrent systemic therapy. Patient and disease characteristics associated with disease control and clinical outcomes were analyzed by Cox proportional hazards regression and Kaplan-Meier analyses. Imaging at the time of first failure was used to categorize failure patterns.
RESULTS: We identified 134 patients with a median follow-up of 56.2 months (range 8.2-160.2 months). Local and regional control at 5 years was 91.5% (95% CI: 86.8-96.4%), and 90.8% (95% CI: 85.6-96.2%), respectively. Of the 14 locoregional failures, there were 10 in-field (Type A), 3 marginal (Type B), and 1 geographic (Type E). Age >70 years (HR 5.42; 95% CI: 1.87-15.68) and T4 versus T1-3 (HR 4.09; 95% CI: 1.01-2.65) were associated with increased rates of locoregional failure on multivariate analysis. The rate of gastrostomy tube retention at one year was 6.0% (range 2.8-12.7%).
CONCLUSIONS: Management of patients with p16-positive oropharyngeal squamous cell carcinoma using definitive radiotherapy and a high-dose planning target volume created without a gross tumor volume to clinical tumor volume expansion resulted in high locoregional control with the vast majority of failures occurring within the high-dose field. These data warrant prospective evaluation of this technique as a therapy de-intensification approach.
PMID:31010623 | PMC:PMC7062456 | DOI:10.1016/j.oraloncology.2019.03.013
View details for PubMedID 31010623
-
More
-
The ASTRO Research Portfolio: Where Do We Go From Here? International journal of radiation oncology, biology, physics
Yu JB, Beck TF, Anscher MS, Baschnagel AM, Brock KK, Carlson DJ, Dominello MM, Kimple RJ, Knisely PS, Mendonca MS, Mian OY, Singh AK, Moros EG, Keen JC
2019 Feb 1;103(2):308-309. doi: 10.1016/j.ijrobp.2018.09.009.
-
Analysis of the 2017 American Society for Radiation Oncology (ASTRO) Research Portfolio International journal of radiation oncology, biology, physics
Yu JB, Beck TF, Anscher MS, Baschnagel AM, Brock KK, Carlson DJ, Dominello MM, Kimple RJ, Knisely JP, Mendonca MS, Mian OY, Singh AK, Moros EG, Keen JC
2019 Feb 1;103(2):297-304. doi: 10.1016/j.ijrobp.2018.07.2056.
-
More
PURPOSE: Research in radiation oncology (RO) is imperative to support the discovery of new uses of radiation and improvement of current approaches to radiation delivery and to foster the continued evolution of our field. Therefore, in 2016, the American Society of Radiation Oncology performed an evaluation of research grant funding for RO.
METHODS AND MATERIALS: Members of the Society of Chairs of Academic Radiation Oncology Programs (SCAROP) were asked about funded and unfunded grants that were submitted by their departments between the fiscal years 2014 and 2016. Grants were grouped according to broad categories defined by the 2017 American Society of Radiation Oncology Research Agenda. Additionally, active grants in the National Institutes of Health (NIH) Research Portfolio Online Reporting Tools database were collated using RO faculty names.
RESULTS: Overall, there were 816 funded (44%) and 1031 unfunded (56%) SCAROP-reported grants. Total grant funding was over $196 million. The US government funded the plurality (42.2%; 345 of 816) of grants compared with nonprofit and industry funders. Investigators from 10 institutions accounted for >75% of funded grants. Of the funded grants, 43.5% were categorized as "genomic influences and targeted therapies." The proportion of funded to unfunded grants was highest within the category of "tumor microenvironment, normal tissue effects, and reducing toxicity" (53.4% funded). "New clinical trial design and big data" had the smallest share of SCAROP grant applications and the lowest percent funded (38.3% of grants). NIH grants to RO researchers in 2014 to 2016 accounted for $85 million in funding. From the 31 responding SCAROP institutions, there was a 28% average success rate for RO proposals submitted to the NIH during this period.
CONCLUSIONS: Though RO researchers from responding institutions were relatively successful in obtaining funding, the overall amount awarded remains small. Continued advocacy on behalf of RO is needed, as well as investment to make research careers more attractive areas for emerging faculty.
PMID:30647006 | DOI:10.1016/j.ijrobp.2018.07.2056
View details for PubMedID 30647006
-
More
-
Correction: AXL Is a Logical Molecular Target in Head and Neck Squamous Cell Carcinoma Clinical cancer research : an official journal of the American Association for Cancer Research
Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Coan JP, Pearson HE, Bahrar H, Fowler TL, Bednarz BP, Saha S, Yang D, Gill PS, Lingen MW, Saloura V, Villaflor VM, Salgia R, Kimple RJ, Wheeler DL
2018 Dec 1;24(23):6099. doi: 10.1158/1078-0432.CCR-18-3194.
-
HPV impacts survival of stage IVC non-oropharyngeal HNSCC cancer patients Otorhinolaryngology-head and neck surgery
Burr AR, Harari PM, Ko HC, Chen S, Yu M, Baschnagel AM, Kimple RJ, Witek ME
2018;3(1):10.15761/OHNS.1000160. doi: 10.15761/OHNS.1000160. Epub 2018 Feb 24.
-
More
OBJECTIVES: Human papillomavirus (HPV) status is a favorable prognostic marker for patients with oropharyngeal squamous cell carcinoma (OPSCC) and non-metastatic head and neck non-OPSCC. We evaluated the impact of HPV status on overall survival (OS) for patients with Stage IVC non-OPSCC.
MATERIALS AND METHODS: Patients diagnosed with Stage IVC non-OPSCC and known HPV status between 2010-2013 were identified in the National Cancer Database. Univariate and multivariate analyses were performed to determine factors associated with OS. Propensity score-weighted Kaplan-Meier estimation was used to adjust for confounders in OS analyses. Multiple imputation method was used for sensitivity analysis.
RESULTS: We identified 708 patients with Stage IVC non-OPSCC with 30% being HPV-positive. Unadjusted median survival was 10.3 months for HPV-negative patients and 21.4 months for HPV-positive patients (p<0.0001). Age ≥ 65 and tumor diameter were associated with worse OS (p<0.05) while treatment versus no treatment and HPV-positive status were associated with improved OS on multivariate analysis (p<0.001). Adjusted median survival for patients with HPV-negative and HPV-positive disease was 11.1 months and 23.8 months, respectively (p<0.001). On unadjusted subgroup analysis, patients with HPV-positive oral cavity disease exhibited improved outcomes (p<0.0001) while HPV-positive hypopharynx (p<0.06) and larynx (p<0.12) patients exhibited a trend for improved OS compared to HPV-negative patients. The survival advantage associated with HPV positivity was maintained on sensitivity analysis (p<0.01).
CONCLUSION: These data demonstrate a clinically meaningful association between HPV status and OS in patients with non-OSPCC presenting with Stage IVC disease. In the absence of randomized data, these findings support active consideration of HPV status in clinical decision making, clinical trial design, and patient counseling regarding prognosis.
PMID:30271885 | PMC:PMC6157736 | DOI:10.15761/OHNS.1000160
View details for PubMedID 30271885
-
More
-
In Reply to Lin and Golden International journal of radiation oncology, biology, physics
Jang S, Rosenberg SA, Bradley KA, Kimple RJ
2018 Nov 1;102(3):672. doi: 10.1016/j.ijrobp.2018.06.043.
-
MERTK Mediates Intrinsic and Adaptive Resistance to AXL-targeting Agents Molecular cancer therapeutics
McDaniel NK, Cummings CT, Iida M, Hülse J, Pearson HE, Vasileiadi E, Parker RE, Orbuch RA, Ondracek OJ, Welke NB, Kang GH, Davies KD, Wang X, Frye SV, Earp HS, Harari PM, Kimple RJ, DeRyckere D, Graham DK, Wheeler DL
2018 Nov;17(11):2297-2308. doi: 10.1158/1535-7163.MCT-17-1239. Epub 2018 Aug 9.
-
More
The TAM (TYRO3, AXL, MERTK) family receptor tyrosine kinases (RTK) play an important role in promoting growth, survival, and metastatic spread of several tumor types. AXL and MERTK are overexpressed in head and neck squamous cell carcinoma (HNSCC), triple-negative breast cancer (TNBC), and non-small cell lung cancer (NSCLC), malignancies that are highly metastatic and lethal. AXL is the most well-characterized TAM receptor and mediates resistance to both conventional and targeted cancer therapies. AXL is highly expressed in aggressive tumor types, and patients with cancer are currently being enrolled in clinical trials testing AXL inhibitors. In this study, we analyzed the effects of AXL inhibition using a small-molecule AXL inhibitor, a monoclonal antibody (mAb), and siRNA in HNSCC, TNBC, and NSCLC preclinical models. Anti-AXL-targeting strategies had limited efficacy across these different models that, our data suggest, could be attributed to upregulation of MERTK. MERTK expression was increased in cell lines and patient-derived xenografts treated with AXL inhibitors and inhibition of MERTK sensitized HNSCC, TNBC, and NSCLC preclinical models to AXL inhibition. Dual targeting of AXL and MERTK led to a more potent blockade of downstream signaling, synergistic inhibition of tumor cell expansion in culture, and reduced tumor growth in vivo Furthermore, ectopic overexpression of MERTK in AXL inhibitor-sensitive models resulted in resistance to AXL-targeting strategies. These observations suggest that therapeutic strategies cotargeting both AXL and MERTK could be highly beneficial in a variety of tumor types where both receptors are expressed, leading to improved survival for patients with lethal malignancies. Mol Cancer Ther; 17(11); 2297-308. ©2018 AACR.
PMID:30093568 | PMC:PMC6215511 | DOI:10.1158/1535-7163.MCT-17-1239
View details for PubMedID 30093568
-
More
-
Results From 10 Years of a Free Oral Cancer Screening Clinic at a Major Academic Health Center International journal of radiation oncology, biology, physics
Blitzer GC, Rosenberg SA, Anderson BM, McCulloch TM, Wieland AM, Hartig GK, Bruce JY, Witek ME, Kimple RJ, Harari PM
2018 Sep 1;102(1):146-148. doi: 10.1016/j.ijrobp.2018.05.007. Epub 2018 Jul 3.
-
More
PMID:29980415 | PMC:PMC6089656 | DOI:10.1016/j.ijrobp.2018.05.007
View details for PubMedID 29980415
-
More
-
The Resident Individual Development Plan as a Guide for Radiation Oncology Mentorship International journal of radiation oncology, biology, physics
Ko HC, Kimple RJ
2018 Jul 15;101(4):786-788. doi: 10.1016/j.ijrobp.2018.02.153. Epub 2018 Mar 6.
-
More
PMID:29976489 | PMC:PMC6042972 | DOI:10.1016/j.ijrobp.2018.02.153
View details for PubMedID 29976489
-
More
-
Beyond 'charting outcomes' in the radiation oncology match: analysis of self-reported applicant data Medical education online
Jang S, Rosenberg SA, Hullett C, Bradley KA, Kimple RJ
2018 Dec;23(1):1489691. doi: 10.1080/10872981.2018.1489691.
-
More
The Charting Outcomes resource is useful in gauging an applicant's competiveness for a given specialty. However, many variables are not reported in Charting Outcomes that may influence an applicant's ability to match. A significant proportion of applicants record their experiences in an anonymous, self-reported applicant spreadsheet. We analyzed factors associated with a successful match using this dataset to test the hypothesis that research productivity and high academic performance correlates with success rates. A retrospective analysis of "RadOnc Interview Spreadsheet" for the 2015, 2016, and 2017 radiation oncology match was performed. Data were accessed via studentdoctor.net. Board scores, research characteristics, Sub-I participation, and interview invitation rates were available. Mann-Whitney U, Kruskal-Wallis, and chi-square tests were used for statistical analysis. When possible, results were compared to those reported in the National Residency Match Program's "Charting Outcomes" report. A total of 158 applicants were examined for the applicant characteristics. Applicants applied to a median of 61 programs and received a median of 14 interviews. The mean step 1 score was 248 (range: 198 to 272) and most were in the highest grade point average quartile (68.3%). 21.7% participated in additional research year(s), and 19% obtained a PhD. The majority of applicants took three radiation oncology electives (48.7%). On multivariate analysis, alpha-omega-alpha (AOA) honors society status (p=0.033), participating in a research year (p=0.001) and number of journal publications (p=0.047) significantly correlated with higher interview invitation rates. In summary, this study identifies important considerations for radiation oncology applicants that have not been previously reported, such as induction into AOA and number of journal publications.
PMID:29943670 | PMC:PMC6022246 | DOI:10.1080/10872981.2018.1489691
View details for PubMedID 29943670
-
More
-
Corrigendum: Potential role of the glycolytic oscillator in acute hypoxia in tumors (2015 <em>Phys. Med. Biol.</em> 60 9215) Physics in medicine and biology
Fru LC, Adamson EB, Campos DD, Fain SB, Jacques SL, Kogel vd, Nickel KP, Song C, Kimple RJ, Kissick MW
2018 Jun 6. doi: 10.1088/1361-6560/aacaba. Online ahead of print.
-
More
At the time of publication, our group had performed short tandem repeat (STR) testing on the SCC22B cell line and believed that had been correctly identified. As part of a recent comprehensive process to confirm the identity of cell lines in use in our lab, we repeated STR testing on all cell lines. These results were compared to the ExPASy Cellosaurus database (http://web.expasy.org/cellosaurus/). One cell line used in this manuscript was a near perfect match for T24 (CVCL_0554), a bladder carcinoma cell line commonly found as a cellular contaminant. Although we are unable to test the exact cells used in this manuscript, we believe that the cells labeled as SCC22B are most likely to actually be T24. The authors believe that neither the results nor the conclusions have been significantly changed on the basis of the specific cell line utilized.
PMID:29873307 | DOI:10.1088/1361-6560/aacaba
View details for PubMedID 29873307
-
More
-
In Reply to Hamstra International journal of radiation oncology, biology, physics
Rosenberg SA, Bradley KA, Kimple RJ
2018 Apr 1;100(5):1293-1294. doi: 10.1016/j.ijrobp.2018.01.038.
-
Identity Crisis - Rigor and Reproducibility in Human Cell Lines Radiation research
Eckers JC, Swick AD, Kimple RJ
2018 Jun;189(6):551-552. doi: 10.1667/RR15086.1. Epub 2018 Apr 13.
-
Genomics Reloaded: Rise of the Expression Profiles International journal of radiation oncology, biology, physics
Gan GN, Kimple RJ
2018 May 1;101(1):1-3. doi: 10.1016/j.ijrobp.2017.10.023.
-
More
PMID:29619961 | PMC:PMC6821516 | DOI:10.1016/j.ijrobp.2017.10.023
View details for PubMedID 29619961
-
More
-
Impact of HPV Status on the Prognostic Potential of the AJCC Staging System for Larynx Cancer Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery
Davidson SM, Ko HC, Harari PM, Wieland AM, Chen S, Baschnagel AM, Kimple RJ, Witek ME
2018 Sep;159(3):456-465. doi: 10.1177/0194599818766035. Epub 2018 Apr 3.
-
More
Objective We evaluated the ability of the American Joint Committee on Cancer (AJCC) seventh edition staging system to prognosticate the overall survival of patients with human papillomavirus (HPV)-positive laryngeal squamous cell carcinoma. Study Design Retrospective analysis. Setting National Cancer Database. Subjects and Methods Patients diagnosed with laryngeal squamous cell carcinoma who were treated with curative intent were identified in the National Cancer Database. Multivariate analysis was utilized to determine factors correlated with overall survival in the HPV-negative and HPV-positive cohorts. Unadjusted and propensity score-weighted Kaplan-Meier estimation was used to determine overall survival of HPV-negative and HPV-positive patients across AJCC stage groupings. Results We identified 3238 patients with laryngeal squamous cell carcinoma, of which 2812 were HPV negative and 426 were HPV positive. Overall survival adjusted for age, sex, and comorbidity status confirmed significant differences among all consecutive stage groupings (I vs II, P < .001; II vs III, P < .05; III vs IVA, P < .001; IVA vs IVB, P < .05) in the HPV-negative cohort, whereas only stages IVAs and IVB ( P < .01) exhibited a significant difference in overall survival for HPV-positive patients. Conclusion The current AJCC staging system does not accurately distinguish risk of mortality for patients with HPV-positive disease. These data support the consideration of HPV status in estimating prognosis as well as clinical trial design and clinical decision making for patients with laryngeal squamous cell carcinoma.
PMID:29611770 | PMC:PMC7141595 | DOI:10.1177/0194599818766035
View details for PubMedID 29611770
-
More
-
Value of Elective Radiation Oncology Rotations: How Many Is Too Many? International journal of radiation oncology, biology, physics
Jang S, Rosenberg SA, Hullet C, Bradley KA, Kimple RJ
2018 Mar 1;100(3):558-559. doi: 10.1016/j.ijrobp.2017.10.052. Epub 2017 Nov 6.
-
More
PMID:29413271 | PMC:PMC5806148 | DOI:10.1016/j.ijrobp.2017.10.052
View details for PubMedID 29413271
-
More
-
Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report and review of the literature Clinical neurology and neurosurgery
Gallagher SJ, Rosenberg SA, Francis D, Salamat S, Howard SP, Kimple RJ
2018 Feb;165:76-80. doi: 10.1016/j.clineuro.2017.12.014. Epub 2018 Jan 6.
-
More
Primary leiomyosarcoma is a rare tumor in the CNS, with few reported cases. Here, we describe a case of a primary intracranial leiomyosarcoma of the tentorium cerebelli. A 43-year-old woman presented with headache, acute vision loss, and difficulty speaking. MRI revealed a large heterogeneous-enhancing occipital mass, which was subsequently resected and diagnosed as a primary intracranial leiomyosarcoma. The patient went onto adjuvant radiotherapy delivering 60 Gy in 30 fractions. These tumors are exceedingly rare in immunocompetent individuals. We reviewed the 16 cases that have been reported in the literature. Surgical resection was the most common treatment (92%) with 53% receiving adjuvant radiation. There currently is no standard treatment regimen for intracranial leiomyosarcomas. Additional case reports that include descriptive treatment approaches with patient outcomes may help ascertain the best approach to treating these malignancies.
PMID:29324399 | DOI:10.1016/j.clineuro.2017.12.014
View details for PubMedID 29324399
-
More
-
Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma Cell death & disease
Redlich N, Robinson AM, Nickel KP, Stein AP, Wheeler DL, Adkins DR, Uppaluri R, Kimple RJ, Van Tine A, Michel LS
2018 Jan 5;9(1):5. doi: 10.1038/s41419-017-0029-0.
-
More
ErbB3 has been widely implicated in treatment resistance, but its role as a primary treatment target is less clear. Canonically ErbB3 requires EGFR or ErbB2 for activation, whereas these two established treatment targets are thought to signal independently of ErbB3. In this study, we show that ErbB3 is essential for tumor growth of treatment-naive HNSCC patient-derived xenografts. This ErbB3 dependency occurs via ErbB3-mediated control of EGFR activation and HIF1α stabilization, which require ErbB3 and its ligand neuregulin-1. Here, we show that ErbB3 antibody treatment selects for a population of ErbB3-persister cells that express high levels of the transmembrane protein Trop2 that we previously identified as an inhibitor of ErbB3. Co-treatment with anti-ErbB3 and anti-Trop2 antibodies is synergistic and produces a greater anti-tumor response than either antibody alone. Collectively, these data both compel a revision of ErbB-family signaling and delineate a strategy for its effective inhibition in HNSCC.
PMID:29305574 | PMC:PMC5849045 | DOI:10.1038/s41419-017-0029-0
View details for PubMedID 29305574
-
More
-
Biological characterization of a novel in vitro cell irradiator PloS one
Fowler TL, Fisher MM, Bailey AM, Bednarz BP, Kimple RJ
2017 Dec 12;12(12):e0189494. doi: 10.1371/journal.pone.0189494. eCollection 2017.
-
More
To evaluate the overall robustness of a novel cellular irradiator we performed a series of well-characterized, dose-responsive assays to assess the consequences of DNA damage. We used a previously described novel irradiation system and a traditional 137Cs source to irradiate a cell line. The generation of reactive oxygen species was assessed using chloromethyl-H2DCFDA dye, the induction of DNA DSBs was observed using the comet assay, and the initiation of DNA break repair was assessed through γH2AX image cytometry. A high correlation between physical absorbed dose and biologic dose was seen for the production of intracellular reactive oxygen species, physical DNA double strand breaks, and modulation of the cellular double stand break pathway. The results compared favorably to irradiation with a traditional 137Cs source. The rapid, straightforward tests described form a reasonable approach for biologic characterization of novel irradiators. These additional testing metrics go beyond standard physics testing such as Monte Carlo simulation and thermo-luminescent dosimeter evaluation to confirm that a novel irradiator can produce the desired dose effects in vitro. Further, assessment of these biological metrics confirms that the physical handling of the cells during the irradiation process results in biologic effects that scale appropriately with dose.
PMID:29232400 | PMC:PMC5726654 | DOI:10.1371/journal.pone.0189494
View details for PubMedID 29232400
-
More
-
The Second Stain: A Viral Whodunnit International journal of radiation oncology, biology, physics
Rosenberg SA, Kimple RJ
2017 Dec 1;99(5):1061. doi: 10.1016/j.ijrobp.2017.05.027.
-
The Future of Radiobiology Journal of the National Cancer Institute
Kirsch DG, Diehn M, Kesarwala AH, Maity A, Morgan MA, Schwarz JK, Bristow R, Demaria S, Eke I, Griffin RJ, Haas-Kogan D, Higgins GS, Kimmelman AC, Kimple RJ, Lombaert IM, Ma L, Marples B, Pajonk F, Park CC, Schaue D, Tran PT, Willers H, Wouters BG, Bernhard EJ
2018 Apr 1;110(4):329-340. doi: 10.1093/jnci/djx231.
-
More
Innovation and progress in radiation oncology depend on discovery and insights realized through research in radiation biology. Radiobiology research has led to fundamental scientific insights, from the discovery of stem/progenitor cells to the definition of signal transduction pathways activated by ionizing radiation that are now recognized as integral to the DNA damage response (DDR). Radiobiological discoveries are guiding clinical trials that test radiation therapy combined with inhibitors of the DDR kinases DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia mutated (ATM), ataxia telangiectasia related (ATR), and immune or cell cycle checkpoint inhibitors. To maintain scientific and clinical relevance, the field of radiation biology must overcome challenges in research workforce, training, and funding. The National Cancer Institute convened a workshop to discuss the role of radiobiology research and radiation biologists in the future scientific enterprise. Here, we review the discussions of current radiation oncology research approaches and areas of scientific focus considered important for rapid progress in radiation sciences and the continued contribution of radiobiology to radiation oncology and the broader biomedical research community.
PMID:29126306 | PMC:PMC5928778 | DOI:10.1093/jnci/djx231
View details for PubMedID 29126306
-
More
-
Overcoming Resistance to Cetuximab with Honokiol, A Small-Molecule Polyphenol Molecular cancer therapeutics
Pearson HE, Iida M, Orbuch RA, McDaniel NK, Nickel KP, Kimple RJ, Arbiser JL, Wheeler DL
2018 Jan;17(1):204-214. doi: 10.1158/1535-7163.MCT-17-0384. Epub 2017 Oct 20.
-
More
Overexpression and activation of the EGFR have been linked to poor prognosis in several human cancers. Cetuximab is a mAb against EGFR that is used for the treatment in head and neck squamous cell carcinoma (HNSCC) and metastatic colorectal cancer. Unfortunately, most tumors have intrinsic or will acquire resistance to cetuximab during the course of therapy. Honokiol is a natural compound found in the bark and leaves of the Chinese Magnolia tree and is established to have several anticancer properties without appreciable toxicity. In this study, we hypothesized that combining cetuximab and honokiol treatments could overcome acquired resistance to cetuximab. We previously developed a model of acquired resistance to cetuximab in non-small cell lung cancer H226 cell line. Treatment of cetuximab-resistant clones with honokiol and cetuximab resulted in a robust antiproliferative response. Immunoblot analysis revealed the HER family and their signaling pathways were downregulated after combination treatment, most notably the proliferation (MAPK) and survival (AKT) pathways. In addition, we found a decrease in phosphorylation of DRP1 and reactive oxygen species after combination treatment in cetuximab-resistant clones, which may signify a change in mitochondrial function. Furthermore, we utilized cetuximab-resistant HNSCC patient-derived xenografts (PDX) to test the benefit of combinatorial treatment in vivo There was significant growth delay in PDX tumors after combination treatment with a subsequent downregulation of active MAPK, AKT, and DRP1 signaling as seen in vitro Collectively, these data suggest that honokiol is a promising natural compound in overcoming acquired resistance to cetuximab. Mol Cancer Ther; 17(1); 204-14. ©2017 AACR.
PMID:29054984 | PMC:PMC5752575 | DOI:10.1158/1535-7163.MCT-17-0384
View details for PubMedID 29054984
-
More
-
Survival Outcomes for Patients With T3N0M0 Squamous Cell Carcinoma of the Glottic Larynx JAMA otolaryngology-- head & neck surgery
Ko HC, Harari PM, Chen S, Wieland AM, Yu M, Baschnagel AM, Kimple RJ, Witek ME
2017 Nov 1;143(11):1126-1133. doi: 10.1001/jamaoto.2017.1756.
-
More
IMPORTANCE: Radiotherapy (RT)-based organ preservation approaches for patients with advanced laryngeal cancer have been established stepwise through prospective randomized clinical trials. However, broad adoption of these approaches has stimulated discussion about long-term results challenging their applicability in a heterogeneous patient population, most recently for patients with T3 disease.
OBJECTIVE: To define outcomes in patients with clinical T3N0M0 glottic laryngeal cancer treated with definitive surgical and RT-based approaches.
DESIGN, SETTING, AND PARTICIPANTS: This retrospective cohort study included patients treated from January 1, 2004, through December 31, 2013, with a median follow-up time of 58 months (range, 0-126.6 months) in the National Cancer Database. Of the 4003 patients with T3N0M0 disease, 2622 received definitive therapy defined by the study protocol. Data were obtained from the clinical oncology database sourced from hospital registry data that are collected from more than 1500 Commission on Cancer-accredited facilities. Data were analyzed from September 14, 2016, through April 24, 2017.
INTERVENTIONS: Radiotherapy, chemoradiotherapy, surgery, surgery and RT, or surgery and chemoradiotherapy.
MAIN OUTCOMES AND MEASURES: Five-year overall survival (OS).
RESULTS: A total of 2622 patients (2251 men [85.9%] and 371 women [14.1%]; median age, 64 years [range, 19-90 years]) were included in the analytic cohort. In the overall patient cohort, the adjusted 5-year survival probability was 53%. No statistical differences were observed between the primary surgery (53%; 95% CI, 48%-57%) and primary RT (54%; 95% CI, 52%-57%) cohorts. In multivariate analysis, patient factors associated with decreased OS included age (hazard ratio [HR], 1.04; 95% CI, 1.03-1.04), insurance status (HR, 1.26; 95% CI, 1.06-1.50), and increasing comorbidity (HR, 1.20; 95% CI, 1.02-1.42).
CONCLUSIONS AND RELEVANCE: Current management of T3N0M0 glottic laryngeal cancer relies largely on RT-based organ preservation approaches. The present study substantiates randomized clinical trial data supporting the use of RT-based organ preservation approaches for patients with T3N0M0 glottic laryngeal cancer without compromising OS.
PMID:29049434 | PMC:PMC5710357 | DOI:10.1001/jamaoto.2017.1756
View details for PubMedID 29049434
-
More
-
Prognostic implications of human papillomavirus status for patients with non-oropharyngeal head and neck squamous cell carcinomas Journal of cancer research and clinical oncology
Ko HC, Harari PM, Sacotte RM, Chen S, Wieland AM, Yu M, Baschnagel AM, Bruce JY, Kimple RJ, Witek ME
2017 Nov;143(11):2341-2350. doi: 10.1007/s00432-017-2481-8. Epub 2017 Jul 27.
-
More
PURPOSE: We examined overall survival in a large cohort of patients with human papillomavirus (HPV)-positive and HPV-negative non-oropharyngeal squamous cell carcinoma of the head and neck (non-OPSCC).
METHODS: Patients diagnosed with non-OPSCC and known HPV status were identified in the National Cancer Database (NCDB). Multivariate logistic regression was applied to examine factors associated with HPV status. Multivariate analysis was utilized to determine factors correlated with overall survival. Propensity score-weighted Kaplan-Meier estimation was used to adjust for confounders in survival analyses. Multiple imputation method was used for sensitivity analysis.
RESULTS: We identified 19,993 non-OPSCC patients with 5070 being positive for HPV in the NCDB. Median follow-up was 23.5 months. HPV-positive patients were more commonly male, white, with a lower comorbidity index score, presenting with T-stage <2, and N-stage ≥1. Unadjusted 3-year overall survival was 62% and 80% for HPV-negative and HPV-positive patients, respectively (p < 0.0001). On multivariate analysis, mortality was reduced for HPV-positive patients with early stage (HR = 0.68) and locally advanced disease (HR = 0.46). Adjusted 3-year overall survival was 65% for HPV-negative and 76% for HPV-positive patients (p < 0.0001). The survival advantage of HPV was maintained in all subsites and robust on sensitivity analysis.
CONCLUSIONS: Patients with HPV-positive non-OPSCC exhibit similar characteristics as HPV-positive OPSCC. Overall survival was significantly higher for patients with HPV-positive versus HPV-negative non-OPSCC. These data reveal that HPV-positive non-OPSCC represent a favorable cohort that warrants recognition in the design of future clinical trial investigation.
PMID:28752235 | PMC:PMC7069668 | DOI:10.1007/s00432-017-2481-8
View details for PubMedID 28752235
-
More
-
Radiosensitization of Adenoid Cystic Carcinoma with MDM2 Inhibition Clinical cancer research : an official journal of the American Association for Cancer Research
Prabakaran PJ, Javaid AM, Swick AD, Werner LR, Nickel KP, Sampene E, Hu R, Ong IM, Bruce JY, Hartig GK, Wieland AM, Canon J, Harari PM, Kimple RJ
2017 Oct 15;23(20):6044-6053. doi: 10.1158/1078-0432.CCR-17-0969. Epub 2017 Jun 28.
-
More
Purpose: Adenoid cystic carcinoma (ACC) is a rare cancer arising from the major or minor salivary gland tissues of the head and neck. There are currently no approved systemic agents or known radiosensitizers for ACC. Unlike the more common head and neck squamous cell carcinomas that frequently harbor TP53 mutations, ACCs contain TP53 mutations at a rate of <5%, rendering them an attractive target for MDM2 inhibition.Experimental Design: We report the successful establishment and detailed characterization of a TP53-WT ACC patient-derived xenograft (PDX), which retained the histologic features of the original patient tumor. We evaluated this model for response to the MDM2 inhibitor AMG 232 as monotherapy and in combination with radiotherapy.Results: AMG 232 monotherapy induced modest tumor growth inhibition, and radiation monotherapy induced a transient tumor growth delay in a dose-dependent fashion. Strikingly, combination treatment of AMG 232 with radiotherapy (including low-dose radiotherapy of 2 Gy/fraction) induced dramatic tumor response and high local tumor control rates 3 months following treatment. Posttreatment analysis revealed that although both AMG 232 and radiotherapy alone induced TP53 tumor-suppressive activities, combination therapy amplified this response with potent induction of apoptosis after combination treatment.Conclusions: These data identify that MDM2 inhibition can provide potent radiosensitization in TP53-WT ACC. In light of the absence of effective systemic agents for ACC, the powerful response profile observed here suggests that clinical trial evaluation of this drug/radiotherapy combination may be warranted to improve local control in this challenging malignancy. Clin Cancer Res; 23(20); 6044-53. ©2017 AACR.
PMID:28659312 | PMC:PMC5641244 | DOI:10.1158/1078-0432.CCR-17-0969
View details for PubMedID 28659312
-
More
-
Small cell carcinoma of the head and neck: An analysis of the National Cancer Database Oral oncology
Pointer KB, Ko HC, Brower JV, Witek ME, Kimple RJ, Lloyd RV, Harari PM, Baschnagel AM
2017 Jun;69:92-98. doi: 10.1016/j.oraloncology.2017.04.009. Epub 2017 Apr 25.
-
More
PURPOSE/OBJECTIVE(S): To evaluate treatment trends and overall survival of patients with small cell carcinoma of the head and neck region.
MATERIALS/METHODS: Patients from 2004 to 2012 were identified from the National Cancer Database. Patient demographics and overall survival were analyzed. Multivariable analysis was used to identify predictors of survival.
RESULTS: Among 347,252 head and neck patients a total of 1042 (0.3%) patients with small cell carcinoma were identified. 17% of patients were diagnosed as stage I/II, 61% as stage III/IVA/IVB and 22% as stage IVC disease. The distribution by anatomic site was 9% oral cavity, 12% oropharynx, 35% larynx, 4% hypopharynx, 10% nasopharynx and 30% nasal cavity and paranasal sinuses. The median overall survival by anatomical site was 20.8months for oral cavity, 23.7months for oropharynx, 17.9months for larynx/hypopharynx, 15.1months for nasopharynx and 36.4months for nasal cavity primary tumors. On multivariable analysis across stage, patients with nasal cavity and paranasal sinuses tumors had the best survival and patients with nasopharynx primaries had the worst survival. In stage I/II patients, type of treatment delivered resulted in no overall survival difference (p=0.78). In patients with locally advanced disease, there was no difference in survival between those treated with combined surgery, radiotherapy and chemotherapy compared to those treated only with radiotherapy and chemotherapy (p=0.46). The addition of radiotherapy to chemotherapy in the metastatic setting did not result in improved survival (p=0.14).
CONCLUSIONS: Small cell carcinoma of the head and neck is a rare malignancy with a poor prognosis. The addition of surgery to radiotherapy and chemotherapy did not improve survival in patients with locally advanced disease.
PMID:28559027 | PMC:PMC5553627 | DOI:10.1016/j.oraloncology.2017.04.009
View details for PubMedID 28559027
-
More
-
Clinical Outcomes and Prognostic Factors of Adenoid Cystic Carcinoma of the Head and Neck Anticancer research
Jang S, Patel PN, Kimple RJ, McCulloch TM
2017 Jun;37(6):3045-3052. doi: 10.21873/anticanres.11659.
-
More
BACKGROUND: Adenoid cystic carcinoma (ACC) is a salivary gland malignancy with unpredictable growth and poorly understood prognostic factors.
PATIENTS AND METHODS: A database search of patients treated at a single Institution was used to identify patients with histologically-confirmed ACC. Patient, tumor, and treatment characteristics were examined via review of medical records.
RESULTS: Overall survival of 70 patients identified at 5, 10, and 15 years was 80.4%, 61.3%, and 29.4%, respectively. Disease recurrence was seen in 31.9%; of these, 72.7% developed distant metastasis. Older age, higher stage, skull base involvement, positive margins, and metastatic disease, but not local recurrence, predicted a worse overall survival. Higher stage and skull base disease were also associated with shorter disease-free survival. While lung metastasis was the most common, vertebral metastasis was associated with poorer survival.
CONCLUSION: Disease stage, positive margins, skull base involvement, perineural invasion, time to recurrence, and location of metastasis, but not nodal involvement, could serve as poor prognostic factors in ACC.
PMID:28551643 | PMC:PMC7238770 | DOI:10.21873/anticanres.11659
View details for PubMedID 28551643
-
More
-
Potential Mechanisms of Vascular Thrombosis after Microwave Ablation in an in Vivo Liver Journal of vascular and interventional radiology : JVIR
Chiang J, Nickel K, Kimple RJ, Brace CL
2017 Jul;28(7):1053-1058. doi: 10.1016/j.jvir.2017.03.034. Epub 2017 Apr 26.
-
More
PURPOSE: To evaluate potential biologic and thermal mechanisms of the observed differences in thrombosis rates between hepatic vessels during microwave (MW) ablation procedures.
MATERIALS AND METHODS: MW ablation antennae were placed in single liver lobes of 2 in vivo porcine liver models (n = 3 in each animal; N = 6 total) in the proximity of a large (> 5 mm) portal vein (PV) and hepatic veins (HVs). Each ablation was performed with 100 W for 5 minutes. Conventional ultrasound imaging and intravascular temperature probes were used to evaluate vessel patency and temperature changes during the ablation procedure. Vascular endothelium was harvested 1 hour after ablation and used to characterize genes and proteins associated with thrombosis in PVs and HVs.
RESULTS: Targeted PVs within the MW ablation zone exhibited thrombosis at a significantly higher rate than HVs (54.5% vs 0.0%; P = .0046). There was a negligible change in intravascular temperature in PVs and HVs during the ablation procedure (0.2°C ± 0.4 vs 0.6°C ± 0.9; P = .46). PVs exhibited significantly higher gene expression than HVs in terms of fold differences in thrombomodulin (2.9 ± 2.0; P = .0001), von Willebrand factor (vWF; 7.6 ± 1.5; P = .0001), endothelial protein C receptor (3.50 ± 0.49; P = .0011), and plasminogen activator inhibitor (1.46 ± 0.05; P = .0014). Western blot analysis showed significantly higher expression of vWF (2.32 ± 0.92; P = .031) in PVs compared with HVs.
CONCLUSIONS: Large PVs exhibit thrombosis more frequently than HVs during MW ablation procedures. Biologic differences in thrombogenicity, rather than heat transfer, between PVs and HVs may contribute to their different rates of thrombosis.
PMID:28456355 | PMC:PMC5483190 | DOI:10.1016/j.jvir.2017.03.034
View details for PubMedID 28456355
-
More
-
Cotargeting mTORC and EGFR Signaling as a Therapeutic Strategy in HNSCC Molecular cancer therapeutics
Swick AD, Prabakaran PJ, Miller MC, Javaid AM, Fisher MM, Sampene E, Ong IM, Hu R, Iida M, Nickel KP, Bruce JY, Wheeler DL, Kimple RJ
2017 Jul;16(7):1257-1268. doi: 10.1158/1535-7163.MCT-17-0115. Epub 2017 Apr 26.
-
More
Head and neck squamous cell carcinomas (HNSCC) are frequently altered along the PI3K/AKT/mTORC signaling axis. Despite excellent preclinical data, the use of compounds targeting this pathway as monotherapy has been underwhelming in initial clinical trials, and identification of predictive biomarkers remains challenging. To investigate mTORC-specific inhibition, we tested catalytic mTORC (AZD8055) and PI3K/mTORC (NVP-BEZ-235) inhibitors ± cetuximab in a panel of HNSCC cell lines and patient-derived xenografts (PDX). Cell lines were assayed for response to all agents and siRNA knockdown of targets by multiple approaches. All cell lines showed similar response to both drug and siRNA inhibition of both PI3K and mTORC pathways, with anti-EGFR combination producing modest additive effect. Five PDX models that presented PIK3CA mutation or intrinsic cetuximab resistance were treated with a combination of cetuximab and AZD8055. In vivo single-agent mTORC inhibition inhibited growth of one PIK3CA-mutant cancer, but had little effect on any PIK3CAWT or a second PIK3CA-mutant model. In all models, the combination therapy showed greater growth delay than monotherapy. The uniform ability of PI3K and mTORC inhibition to suppress the growth of HNSCC cells highlights the pathway's role in driving proliferation. Although single-agent therapy was largely ineffective in vivo, improved response of combination treatment in an array of PDXs suggests the potential for adding a catalytic mTORC inhibitor to cetuximab therapy. Overall, these results add to a growing body of evidence, suggesting that approaches that attempt to match biomarkers to the optimal therapy in HNSCC remain complex and challenging. Mol Cancer Ther; 16(7); 1257-68. ©2017 AACR.
PMID:28446642 | PMC:PMC5505754 | DOI:10.1158/1535-7163.MCT-17-0115
View details for PubMedID 28446642
-
More
-
RETRACTED: The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor Science signaling
Brand TM, Iida M, Corrigan KL, Braverman CM, Coan JP, Flanigan BG, Stein AP, Salgia R, Rolff J, Kimple RJ, Wheeler DL
2017 Jan 3;10(460):eaag1064. doi: 10.1126/scisignal.aag1064.
-
More
The epidermal growth factor receptor (EGFR) is a therapeutic target in patients with various cancers. Unfortunately, resistance to EGFR-targeted therapeutics is common. Previous studies identified two mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Nuclear translocation of EGFR bypasses the inhibitory effects of cetuximab, and the receptor tyrosine kinase AXL mediates cetuximab resistance by maintaining EGFR activation and downstream signaling. Thus, we hypothesized that AXL mediated the nuclear translocation of EGFR in the setting of cetuximab resistance. Cetuximab-resistant clones of non-small cell lung cancer in culture and patient-derived xenografts in mice had increased abundance of AXL and nuclear EGFR (nEGFR). Cellular fractionation analysis, super-resolution microscopy, and electron microscopy revealed that genetic loss of AXL reduced the accumulation of nEGFR. SRC family kinases (SFKs) and HER family ligands promote the nuclear translocation of EGFR. We found that AXL knockdown reduced the expression of the genes encoding the SFK family members YES and LYN and the ligand neuregulin-1 (NRG1). AXL knockdown also decreased the interaction between EGFR and the related receptor HER3 and accumulation of HER3 in the nucleus. Overexpression of LYN and NRG1 in cells depleted of AXL resulted in accumulation of nEGFR, rescuing the deficit induced by lack of AXL. Collectively, these data uncover a previously unrecognized role for AXL in regulating the nuclear translocation of EGFR and suggest that AXL-mediated SFK and NRG1 expression promote this process.
PMID:28049763 | PMC:PMC7094775 | DOI:10.1126/scisignal.aag1064
View details for PubMedID 28049763
-
More
-
Defining the boundaries and expanding the utility of head and neck cancer patient derived xenografts Oral oncology
Swick AD, Stein AP, McCulloch TM, Hartig GK, Ong IM, Sampene E, Prabakaran PJ, Liu CZ, Kimple RJ
2017 Jan;64:65-72. doi: 10.1016/j.oraloncology.2016.11.017. Epub 2016 Dec 8.
-
More
BACKGROUND: Patient derived xenografts (PDXs) represent an essential tool in oncologic research, and we sought to further expand our repertoire of head and neck squamous cell carcinoma (HNSCC) while determining potential boundaries for this system.
METHODS: We consented new patients for PDX development and determined if a 24-h time delay from tumor excision to xenograft implantation affected PDX establishment. We developed a tissue microarray (TMA) from formalin fixed, paraffin embedded PDXs and their subsequent passages and carried out quantitative immunohistochemistry for EGFR, pEGFR, pAkt, pERK and ERCC1. First and last passaged PDXs were compared via a paired t-test to examine for the stability of protein expression across passages. We performed a similar comparison of the mutational profile of the patient tumor and resulting xenografts using a targeted sequencing approach.
RESULTS: No patient/tumor characteristics influenced PDX take rate and the 24-h time delay from tumor excision to xenograft implantation did not affect PDX establishment, growth or histology. There was no significant difference in biomarker expression between the first and last passaged PDXs for EGFR, pEGFR, pAkt, and ERCC1. For pERK there was a significant difference (p=0.002), but further analysis demonstrated this only arose in three of 15 PDXs. Targeted sequencing revealed striking stability of passenger and likely driver mutations from patient to xenograft.
CONCLUSIONS: The stability of protein expression across PDX passages will hopefully allow greater investigation of predictive biomarkers in order to identify ones for further pre-clinical and clinical investigation.
PMID:28024726 | PMC:PMC5218527 | DOI:10.1016/j.oraloncology.2016.11.017
View details for PubMedID 28024726
-
More
-
Online patient information from radiation oncology departments is too complex for the general population Practical radiation oncology
Rosenberg SA, Francis DM, Hullet CR, Morris ZS, Brower JV, Anderson BM, Bradley KA, Bassetti MF, Kimple RJ
2017 Jan-Feb;7(1):57-62. doi: 10.1016/j.prro.2016.07.008. Epub 2016 Aug 1.
-
More
PURPOSE: Nearly two-thirds of cancer patients seek information about their diagnosis online. We assessed the readability of online patient education materials found on academic radiation oncology department Web sites to determine whether they adhered to guidelines suggesting that information be presented at a sixth-grade reading level.
METHODS AND MATERIALS: The Association of American Medical Colleges Web site was used to identify all academic radiation oncology departments in the United States. One-third of these department Web sites were selected for analysis using a random number generator. Both general information on radiation therapy and specific information regarding various radiation modalities were collected. To test the hypothesis that the readability of these online educational materials was written at the recommended grade level, a panel of 10 common readability tests was used. A composite grade level of readability was constructed using the 8 readability measures that provide a single grade-level output.
RESULTS: A mean of 5605 words (range, 2058-12,837) from 30 department Web sites was collected. Using the composite grade level score, the overall mean readability level was determined to be 13.36 (12.83-13.89), corresponding to a collegiate reading level. This was significantly higher than the target sixth-grade reading level (middle school, t (29) = 27.41, P < .001).
CONCLUSIONS: Online patient educational materials from academic radiation oncology Web sites are significantly more complex than recommended by the National Institutes of Health and the Department of Health and Human Services. To improve patients' comprehension of radiation therapy and its role in their treatment, our analysis suggests that the language used in online patient information should be simplified to communicate the information at a more appropriate level.
PMID:27663932 | PMC:PMC5219938 | DOI:10.1016/j.prro.2016.07.008
View details for PubMedID 27663932
-
More
-
Readability of Online Patient Educational Resources Found on NCI-Designated Cancer Center Web Sites Journal of the National Comprehensive Cancer Network : JNCCN
Rosenberg SA, Francis D, Hullett CR, Morris ZS, Fisher MM, Brower JV, Bradley KA, Anderson BM, Bassetti MF, Kimple RJ
2016 Jun;14(6):735-40. doi: 10.6004/jnccn.2016.0075.
-
More
BACKGROUND: The NIH and Department of Health & Human Services recommend online patient information (OPI) be written at a sixth grade level. We used a panel of readability analyses to assess OPI from NCI-Designated Cancer Center (NCIDCC) Web sites.
METHODS: Cancer.gov was used to identify 68 NCIDCC Web sites from which we collected both general OPI and OPI specific to breast, prostate, lung, and colon cancers. This text was analyzed by 10 commonly used readability tests: the New Dale-Chall Readability Formula, Flesch Reading Ease scale, Flesch-Kinaid Grade Level, FORCAST scale, Fry Readability Graph, Simple Measure of Gobbledygook test, Gunning Frequency of Gobbledygook index, New Fog Count, Raygor Readability Estimate Graph, and Coleman-Liau Index. We tested the hypothesis that the readability of NCIDCC OPI was written at the sixth grade level. Secondary analyses were performed to compare readability of OPI between comprehensive and noncomprehensive centers, by region, and to OPI produced by the American Cancer Society (ACS).
RESULTS: A mean of 30,507 words from 40 comprehensive and 18 noncomprehensive NCIDCCs was analyzed (7 nonclinical and 3 without appropriate OPI were excluded). Using a composite grade level score, the mean readability score of 12.46 (ie, college level: 95% CI, 12.13-12.79) was significantly greater than the target grade level of 6 (middle-school: P<.001). No difference between comprehensive and noncomprehensive centers was identified. Regional differences were identified in 4 of the 10 readability metrics (P<.05). ACS OPI provides easier language, at the seventh to ninth grade level, across all tests (P<.01).
CONCLUSIONS: OPI from NCIDCC Web sites is more complex than recommended for the average patient.
PMID:27283166 | PMC:PMC7236813 | DOI:10.6004/jnccn.2016.0075
View details for PubMedID 27283166
-
More
-
Radiation Promptly Alters Cancer Live Cell Metabolic Fluxes: An In Vitro Demonstration Radiation research
Campos D, Peeters W, Nickel K, Burkel B, Bussink J, Kimple RJ, Kogel vd, Eliceiri KW, Kissick MW
2016 May;185(5):496-504. doi: 10.1667/RR14093.1. Epub 2016 Apr 29.
-
More
Quantitative data is presented that shows significant changes in cellular metabolism in a head and neck cancer cell line 30 min after irradiation. A head and neck cancer cell line (UM-SCC-22B) and a comparable normal cell line, normal oral keratinocyte (NOK) were each separately exposed to 10 Gy and treated with a control drug for disrupting metabolism (potassium cyanide; KCN). The metabolic changes were measured live by fluorescence lifetime imaging of the intrinsically fluorescent intermediate metabolite nicotinamide adenosine dinucleotide (NADH) fluorescence; this method is sensitive to the ratio of bound to free NADH. The results indicated a prompt shift in metabolic signature in the cancer cell line, but not in the normal cell line. Control KCN treatment demonstrated expected metabolic fluxes due to mitochondrial disruption. The detected radiation shift in the cancer cells was blunted in the presence of both a radical scavenger and a HIF-1α inhibitor. The HIF-1α abundance as detected by immunohistochemical staining also increased substantially for these cancer cells, but not for the normal cells. This type of live-cell metabolic monitoring could be helpful for future real-time studies and in designing adaptive radiotherapy approaches.
PMID:27128739 | PMC:PMC4882764 | DOI:10.1667/RR14093.1
View details for PubMedID 27128739
-
More
-
Defects in the Fanconi Anemia Pathway in Head and Neck Cancer Cells Stimulate Tumor Cell Invasion through DNA-PK and Rac1 Signaling Clinical cancer research : an official journal of the American Association for Cancer Research
Romick-Rosendale LE, Hoskins EE, Vinnedge MP, Foglesong GD, Brusadelli MG, Potter SS, Komurov K, Brugmann SA, Lambert PF, Kimple RJ, Virts EL, Hanenberg H, Gillison ML, Wells SI
2016 Apr 15;22(8):2062-73. doi: 10.1158/1078-0432.CCR-15-2209. Epub 2015 Nov 24.
-
More
PURPOSE: Head and neck squamous cell carcinoma (HNSCC) remains a devastating disease, and Fanconi anemia (FA) gene mutations and transcriptional repression are common. Invasive tumor behavior is associated with poor outcome, but relevant pathways triggering invasion are poorly understood. There is a significant need to improve our understanding of genetic pathways and molecular mechanisms driving advanced tumor phenotypes, to develop tailored therapies. Here we sought to investigate the phenotypic and molecular consequences of FA pathway loss in HNSCC cells.
EXPERIMENTAL DESIGN: Using sporadic HNSCC cell lines with and without FA gene knockdown, we sought to characterize the phenotypic and molecular consequences of FA deficiency. FA pathway inactivation was confirmed by the detection of classic hallmarks of FA following exposure to DNA cross-linkers. Cells were subjected to RNA sequencing with qRT-PCR validation, followed by cellular adhesion and invasion assays in the presence and absence of DNA-dependent protein kinase (DNA-PK) and Rac1 inhibitors.
RESULTS: We demonstrate that FA loss in HNSCC cells leads to cytoskeletal reorganization and invasive tumor cell behavior in the absence of proliferative gains. We further demonstrate that cellular invasion following FA loss is mediated, at least in part, through NHEJ-associated DNA-PK and downstream Rac1 GTPase activity.
CONCLUSIONS: These findings demonstrate that FA loss stimulates HNSCC cell motility and invasion, and implicate a targetable DNA-PK/Rac1 signaling axis in advanced tumor phenotypes.
PMID:26603260 | PMC:PMC4834270 | DOI:10.1158/1078-0432.CCR-15-2209
View details for PubMedID 26603260
-
More
-
Potential role of the glycolytic oscillator in acute hypoxia in tumors Physics in medicine and biology
Fru LC, Adamson EB, Campos DD, Fain SB, Jacques SL, Kogel vd, Nickel KP, Song C, Kimple RJ, Kissick MW
2015 Dec 21;60(24):9215-25. doi: 10.1088/0031-9155/60/24/9215. Epub 2015 Nov 18.
-
More
Tumor acute hypoxia has a dynamic component that is also, at least partially, coherent. Using blood oxygen level dependent magnetic resonance imaging, we observed coherent oscillations in hemoglobin saturation dynamics in cell line xenograft models of head and neck squamous cell carcinoma. We posit a well-established biochemical nonlinear oscillatory mechanism called the glycolytic oscillator as a potential cause of the coherent oscillations in tumors. These data suggest that metabolic changes within individual tumor cells may affect the local tumor microenvironment including oxygen availability and therefore radiosensitivity. These individual cells can synchronize the oscillations in patches of similar intermediate glucose levels. These alterations have potentially important implications for radiation therapy and are a potential target for optimizing the cancer response to radiation.
PMID:26576743 | PMC:PMC4833657 | DOI:10.1088/0031-9155/60/24/9215
View details for PubMedID 26576743
-
More
-
Pan-HER Inhibitor Augments Radiation Response in Human Lung and Head and Neck Cancer Models Clinical cancer research : an official journal of the American Association for Cancer Research
Francis DM, Huang S, Armstrong EA, Werner LR, Hullett C, Li C, Morris ZS, Swick AD, Kragh M, Lantto J, Kimple RJ, Harari PM
2016 Feb 1;22(3):633-43. doi: 10.1158/1078-0432.CCR-15-1664. Epub 2015 Sep 29.
-
More
PURPOSE: Aberrant regulation of the EGF receptor family (EGFR, HER2, HER3, HER4) contributes to tumorigenesis and metastasis in epithelial cancers. Pan-HER represents a novel molecular targeted therapeutic composed of a mixture of six monoclonal antibodies against EGFR, HER2, and HER3.
EXPERIMENTAL DESIGN: In the current study, we examine the capacity of Pan-HER to augment radiation response across a series of human lung and head and neck cancers, including EGFR inhibitor-resistant cell lines and xenografts.
RESULTS: Pan-HER demonstrates superior antiproliferative and radiosensitizing impact when compared with cetuximab. The mechanisms underlying these effects appear to involve attenuation of DNA damage repair, enhancement of programmed cell death, cell-cycle redistribution, and induction of cellular senescence. Combined treatment of Pan-HER with single or fractionated radiation in human tumor xenografts reveals a potent antitumor and regrowth delay impact compared with Pan-HER or radiation treatment alone.
CONCLUSIONS: These data highlight the capacity of Pan-HER to augment radiation response in lung and head and neck cancer models and support investigation of Pan-HER combined with radiation as a promising clinical therapeutic strategy.
PMID:26420857 | DOI:10.1158/1078-0432.CCR-15-1664
View details for PubMedID 26420857
-
More
-
Modulation of therapeutic sensitivity by human papillomavirus Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Swick AD, Chatterjee A, Costa AD, Kimple RJ
2015 Sep;116(3):342-5. doi: 10.1016/j.radonc.2015.09.002. Epub 2015 Sep 10.
-
More
Human papillomaviruses (HPVs) are small double-stranded DNA viruses that pose significant public health concerns as the causative agent of approximately 5% of worldwide cancers. The HPV oncogenes E6 and E7 play key roles in carcinogenesis. In the last 15years there has been a significant increase in the incidence of HPV-related head and neck cancers arising primarily in the oropharynx. Patients with HPV-positive head and neck cancers (HNCs) have a significantly improved prognosis compared to those with HPV-negative disease. In this review we will discuss data suggesting how HPV oncogenes modulate both the intrinsic radiation sensitivity of HNCs and also have important effects upon the tumor microenvironment. Together, these findings contribute to the improved outcomes seen in patients with HPV-positive HNC.
PMID:26364887 | PMC:PMC4609293 | DOI:10.1016/j.radonc.2015.09.002
View details for PubMedID 26364887
-
More
-
Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Morris ZS, Weichert JP, Saker J, Armstrong EA, Besemer A, Bednarz B, Kimple RJ, Harari PM
2015 Sep;116(3):504-9. doi: 10.1016/j.radonc.2015.06.015. Epub 2015 Jun 26.
-
More
BACKGROUND AND PURPOSE: CLR1404 is a phospholipid ether that exhibits selective uptake and retention in malignant tissues. Radiolabeled CLR1404 enables tumor-specific positron-emission tomography (PET) imaging ((124)I) and targeted delivery of ionizing radiation ((131)I). Here we describe the first preclinical studies of this diapeutic molecule in head and neck cancer (HNC) models.
MATERIAL AND METHODS: Tumor-selective distribution of (124)I-CLR1404 and therapeutic efficacy of (131)I-CLR1404 were tested in HNC cell lines and patient-derived xenograft tumor models. Monte Carlo dose calculations and (124)I-CLR1404 PET/CT imaging were used to examine (131)I-CLR1404 dosimetry in preclinical HNC tumor models.
RESULTS: HNC tumor xenograft studies including patient-derived xenografts demonstrate tumor-selective uptake and retention of (124)I-CLR1404 resulting in a model of highly conformal dose distribution for (131)I-CLR1404. We observe dose-dependent response to (131)I-CLR1404 with respect to HNC tumor xenograft growth inhibition and this effect is maintained together with external beam radiation.
CONCLUSIONS: We confirm the utility of CLR1404 for tumor imaging and treatment of HNC. This promising agent warrants further investigation in a developing phase I trial combining (131)I-CLR1404 with reduced-dose external beam radiation in patients with loco-regionally recurrent HNC.
PMID:26123834 | PMC:PMC4609259 | DOI:10.1016/j.radonc.2015.06.015
View details for PubMedID 26123834
-
More
-
Merkel Cell Carcinoma Analysis of Outcomes: A 30-Year Experience PloS one
Liang E, Brower JV, Rice SR, Buehler DG, Saha S, Kimple RJ
2015 Jun 8;10(6):e0129476. doi: 10.1371/journal.pone.0129476. eCollection 2015.
-
More
BACKGROUND: Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with poor prognosis. Limited data exists to guide treatment decisions. Here we report on our institutional experience and outcomes treating patients with MCC.
METHODS: A database search (1984-2014) of patients treated at the University of Wisconsin Hospital and Clinics was used to identify patients with histologically confirmed MCC. Patient, tumor, and treatment characteristics were examined via review of medical records. Statistical analyses were performed to assess outcomes and associated prognostic factors.
RESULTS: A total of 87 patients with MCC were identified with a median follow-up of 17 months (mean: 38, range: 0-210 months). Two and five-year overall survival rates were 53.9% and 32.8%, respectively. Recurrence was documented in 31.0% of patients (85.2% locoregional, 48.1% distant and 33.3% both). Patients with a history of immunosuppression exhibited significantly worse survival (hazard ratio, 2.01; 95% CI, 1.1-3.7) when compared to immune-competent individuals. The head and neck region was the most common location of primary lesion (N=49) followed by the extremities (N=31). Upper extremity primaries predicted significantly better overall survival (hazard ratio, 0.48; 95% CI, 0.23-0.99) while lower extremity primaries did not have significantly better results (hazard ratio, 0.5; 95% CI, 0.21-1.2) in comparison to head and neck site of primary. Nodal involvement (hazard ratio, 2.95; 95% CI, 1.5-5.79) was also a negative prognostic factor associated with poor overall survival when compared with clinically node negative patients. Primary tumor size > 2 cm (hazard ratio, 1.76; 95% CI, 0.91-3.4) was not associated with survival.
CONCLUSIONS: This study highlights the role of various factors in determining prognosis of Merkel cell carcinoma; history of immunosuppression, nodal involvement, and head/neck primary predicted worse overall survival. These findings suggest that improvements in both distant and locoregionally directed therapies might play an important role in control of MCC and identify areas for future study.
PMID:26053480 | PMC:PMC4460120 | DOI:10.1371/journal.pone.0129476
View details for PubMedID 26053480
-
More
-
Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review Cancer journal (Sudbury, Mass.)
Stein AP, Saha S, Kraninger JL, Swick AD, Yu M, Lambert PF, Kimple RJ
2015 May-Jun;21(3):138-46. doi: 10.1097/PPO.0000000000000115.
-
More
PURPOSE: The global incidence of oropharyngeal squamous cell carcinoma (OPSCC) has been increasing, and it has been proposed that a rising rate of human papillomavirus (HPV)-associated cancers is driving the observed changes in OPSCC incidence. We carried out this systematic review to further examine the prevalence of HPV in OPSCC over time worldwide.
METHODS: A systematic literature search was performed to identify all articles through January 31, 2014, which reported on the prevalence of HPV in OPSCC. Articles that met the inclusion criteria were divided into 4 time frames (pre-1995, 1995-1999, 2000-2004, and 2005 to present) based on the median year of the study's sample collection period. Using a weighted analysis of variance model, we examined the trends of HPV-positivity over time worldwide, in North America, and in Europe.
RESULTS: Our literature search identified 699 unique articles. One hundred seventy-five underwent review of the entire study, and 105 met the inclusion criteria. These 105 articles reported on the HPV prevalence in 9541 OPSCC specimens across 23 nations. We demonstrated significant increases in the percentage change of HPV-positive OPSCCs from pre-1995 to present: 20.6% worldwide (P for trend: P < 0.001), 21.6% in North America (P = 0.013), and 21.5% in Europe (P = 0.033).
CONCLUSIONS: Interestingly, whereas in Europe there was a steady increase in HPV prevalence across all time frames, reaching nearly 50% most recently, in North America HPV prevalence appears to have plateaued over the past decade at about 65%. These findings may have important implications regarding predictions for the future incidence of OPSCC.
PMID:26049691 | PMC:PMC4459520 | DOI:10.1097/PPO.0000000000000115
View details for PubMedID 26049691
-
More
-
The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy Annals of translational medicine
Kimple RJ, Harari PM
2015 May;3(Suppl 1):S14. doi: 10.3978/j.issn.2305-5839.2015.01.37.
-
More
PMID:26046059 | PMC:PMC4437932 | DOI:10.3978/j.issn.2305-5839.2015.01.37
View details for PubMedID 26046059
-
More
-
Human papillomavirus and head and neck cancer International journal of radiation oncology, biology, physics
Kimple RJ, Sher DJ
2015 Jun 1;92(2):196-9. doi: 10.1016/j.ijrobp.2015.01.015.
-
Acute Toxicity From Breast Cancer Radiation Using Helical Tomotherapy With a Simultaneous Integrated Boost Technology in cancer research & treatment
Wojcieszynski AP, Olson AK, Rong Y, Kimple RJ, Yadav P
2016 Apr;15(2):257-65. doi: 10.1177/1533034615574387. Epub 2015 Mar 16.
-
More
PURPOSE: To evaluate 2 simultaneous integrated boost treatment planning techniques using helical tomotherapy for breast conserving therapy with regard to acute skin toxicity and dosimetry.
METHODS: Thirty-two patients were studied. The original approach was for 16 patients and incorporated a directional block of the ipsilateral lung and breast. An additional 16 patients were planned for using a modified approach that incorporates a full block of the ipsilateral lung exclusive of 4 cm around the breast. Dose-volume histograms of targets and critical structures were evaluated. Skin toxicity monitoring was performed throughout treatment and follow-up using the Common Terminology Criteria for Adverse Events.
RESULTS: Treatment was well tolerated with patients receiving a median dose of 59.36 Gy. Of the 16 patients in both groups, 8 had grade 2 erythema immediately after radiation. On 3-week follow-up, 10 and 7 patients in the original and modified groups showed grade 1 erythema. On 3- and 6-month follow-up, both groups had minimal erythema, with all patients having either grade 0 or 1 symptoms. No grade 2 or 3 toxicities were reported. Mean treatment time was 7.5 and 10.4 minutes using the original and modified methods. Adequate dose coverage was achieved using both methods (V95 = 99.5% and 98%). Mean dose to the heart was 10.5 and 1.8 Gy, respectively (P < .01). For right-sided tumors, the original and modified plans yielded a mean of 8.8 and 1.1 Gy (P < .01) versus 11.7 and 2.4 Gy for left-sided tumors (P < .01). The mean dose to the ipsilateral lung was also significantly lower in the modified plans (11.8 vs. 5.0 Gy, P < .01).
CONCLUSIONS: Tomotherapy is capable of delivering homogeneous treatment plans to the whole breast and lumpectomy cavity using simultaneous integrated boost treatment. Using the treatment methods described herein, extremely low doses to critical structures can be achieved without compromising acute skin toxicity.
PMID:25780060 | PMC:PMC7237233 | DOI:10.1177/1533034615574387
View details for PubMedID 25780060
-
More
-
AXL Is a Logical Molecular Target in Head and Neck Squamous Cell Carcinoma Clinical cancer research : an official journal of the American Association for Cancer Research
Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Coan JP, Pearson HE, Bahrar H, Fowler TL, Bednarz BP, Saha S, Yang D, Gill PS, Lingen MW, Saloura V, Villaflor VM, Salgia R, Kimple RJ, Wheeler DL
2015 Jun 1;21(11):2601-12. doi: 10.1158/1078-0432.CCR-14-2648. Epub 2015 Mar 12.
-
More
PURPOSE: Head and neck squamous cell carcinoma (HNSCC) represents the eighth most common malignancy worldwide. Standard-of-care treatments for patients with HNSCC include surgery, radiation, and chemotherapy. In addition, the anti-EGFR monoclonal antibody cetuximab is often used in combination with these treatment modalities. Despite clinical success with these therapeutics, HNSCC remains a difficult malignancy to treat. Thus, identification of new molecular targets is critical.
EXPERIMENTAL DESIGN: In the current study, the receptor tyrosine kinase AXL was investigated as a molecular target in HNSCC using established cell lines, HNSCC patient-derived xenografts (PDX), and human tumors. HNSCC dependency on AXL was evaluated with both anti-AXL siRNAs and the small-molecule AXL inhibitor R428. Furthermore, AXL inhibition was evaluated with standard-of-care treatment regimens used in HNSCC.
RESULTS: AXL was found to be highly overexpressed in several models of HNSCC, where AXL was significantly associated with higher pathologic grade, presence of distant metastases, and shorter relapse-free survival in patients with HNSCC. Further investigations indicated that HNSCC cells were reliant on AXL for cellular proliferation, migration, and invasion. In addition, targeting AXL increased HNSCC cell line sensitivity to chemotherapy, cetuximab, and radiation. Moreover, radiation-resistant HNSCC cell line xenografts and PDXs expressed elevated levels of both total and activated AXL, indicating a role for AXL in radiation resistance.
CONCLUSIONS: This study provides evidence for the role of AXL in HNSCC pathogenesis and supports further preclinical and clinical evaluation of anti-AXL therapeutics for the treatment of patients with HNSCC.
PMID:25767293 | PMC:PMC5032632 | DOI:10.1158/1078-0432.CCR-14-2648
View details for PubMedID 25767293
-
More
-
Xenograft assessment of predictive biomarkers for standard head and neck cancer therapies Cancer medicine
Stein AP, Swick AD, Smith MA, Blitzer GC, Yang RZ, Saha S, Harari PM, Lambert PF, Liu CZ, Kimple RJ
2015 May;4(5):699-712. doi: 10.1002/cam4.387. Epub 2015 Jan 26.
-
More
Head and neck squamous cell carcinoma (HNSCC) remains a challenging cancer to treat with overall 5-year survival on the order of 50-60%. Therefore, predictive biomarkers for this disease would be valuable to provide more effective and individualized therapeutic approaches for these patients. While prognostic biomarkers such as p16 expression correlate with outcome; to date, no predictive biomarkers have been clinically validated for HNSCC. We generated xenografts in immunocompromised mice from six established HNSCC cell lines and evaluated response to cisplatin, cetuximab, and radiation. Tissue microarrays were constructed from pre- and posttreatment tumor samples derived from each xenograft experiment. Quantitative immunohistochemistry was performed using a semiautomated imaging and analysis platform to determine the relative expression of five potential predictive biomarkers: epidermal growth factor receptor (EGFR), phospho-EGFR, phospho-Akt, phospho-ERK, and excision repair cross-complementation group 1 (ERCC1). Biomarker levels were compared between xenografts that were sensitive versus resistant to a specific therapy utilizing a two-sample t-test with equal standard deviations. Indeed the xenografts displayed heterogeneous responses to each treatment, and we linked a number of baseline biomarker levels to response. This included low ERCC1 being associated with cisplatin sensitivity, low phospho-Akt correlated with cetuximab sensitivity, and high total EGFR was related to radiation resistance. Overall, we developed a systematic approach to identifying predictive biomarkers and demonstrated several connections between biomarker levels and treatment response. Despite these promising initial results, this work requires additional preclinical validation, likely involving the use of patient-derived xenografts, prior to moving into the clinical realm for confirmation among patients with HNSCC.
PMID:25619980 | PMC:PMC4430263 | DOI:10.1002/cam4.387
View details for PubMedID 25619980
-
More
-
High-throughput detection of DNA double-strand breaks using image cytometry BioTechniques
Fowler TL, Bailey AM, Bednarz BP, Kimple RJ
2015 Jan 1;58(1):37-9. doi: 10.2144/000114248. eCollection 2015 Jan.
-
More
Assessment of γH2AX expression for studying DNA double-strand break formation is often performed by manual counting of foci using immunofluorescence microscopy, an approach that is laborious and subject to significant foci selection bias. Here we present a novel high-throughput method for detecting DNA double-strand breaks using automated image cytometry assessment of cell average γH2AX immunofluorescence. Our technique provides an expedient, high-throughput, objective, and cost-effective method for γH2AX analysis.
PMID:25605579 | PMC:PMC4331074 | DOI:10.2144/000114248
View details for PubMedID 25605579
-
More
-
Loss of Trop2 causes ErbB3 activation through a neuregulin-1-dependent mechanism in the mesenchymal subtype of HNSCC Oncotarget
Zhang K, Jones L, Lim S, Maher CA, Adkins D, Lewis J, Kimple RJ, Fertig EJ, Chung CH, Van Tine A, Ellis MJ, Herrlich A, Michel LS
2014 Oct 15;5(19):9281-94. doi: 10.18632/oncotarget.2423.
-
More
In head and neck squamous cell cancer (HNSCC), four intrinsic subtypes (or groups) have been identified, and each one possesses a unique biology that will require specific treatment strategies. We previously reported that mesenchymal (group 2) tumors exhibit reduced levels of Trop2 expression. In this study, we investigated the functional role of Trop2 in HNSCC and find that loss results in autocrine activation of the EGFR family member ErbB3 via neuregulin-1. Trop2 localizes to both the cell surface and cytosol of HNSCC cells and forms a complex with neuregulin-1, which is predominantly cytosolic. Inactivation of Trop2 increases the concentration of neuregulin-1 at the cell surface where it is cleaved to activate ErbB3. In primary HNSCC, detection of ErbB3 activation was limited to Trop2 negative tumors. An analysis of the Cancer Genome Atlas (TCGA) HNSCC dataset confirms enrichment for ErbB3 activity in mesenchymal tumors. Notably, Trop2 loss triggers sensitivity to anti-ErbB3 antibodies, which results in reduced proliferation and tumorigenic growth of Trop2 negative HNSCC cancer cells. These results uncover a molecular mechanism by which tumor cells control the amount of cell-surface neuregulin-1 available for cleavage and ErbB3 activation. Moreover, we demonstrate that Trop2 is a potential surrogate biomarker to identify tumors with ErbB3 activation and may therefore respond to anti-ErbB3 therapeutics.
PMID:25238142 | PMC:PMC4253434 | DOI:10.18632/oncotarget.2423
View details for PubMedID 25238142
-
More
-
Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ
2014 Dec;113(3):337-44. doi: 10.1016/j.radonc.2014.08.026. Epub 2014 Sep 9.
-
More
BACKGROUND AND PURPOSE: Patients with human papillomavirus related (HPV+) head and neck cancers (HNCs) demonstrate improved clinical outcomes compared to traditional HPV negative (HPV-) HNC patients. We have recently shown that HPV+ HNC cells are more sensitive to radiation than HPV- HNC cells. However, roles of HPV oncogenes in regulating the response of DNA damage repair remain unknown.
MATERIAL AND METHODS: Using immortalized normal oral epithelial cell lines, HPV+ HNC derived cell lines, and HPV16 E7-transgenic mice we assessed the repair of DNA damage using γ-H2AX foci, single and split dose clonogenic survival assays, and immunoblot. The ability of E7 to modulate expression of proteins associated with DNA repair pathways was assessed by immunoblot.
RESULTS: HPV16 E7 increased retention of γ-H2AX nuclear foci and significantly decreased sublethal DNA damage repair. While phospho-ATM, phospho-ATR, Ku70, and Ku80 expressions were not altered by E7, Rad51 was induced by E7. Correspondingly, HPV+ HNC cell lines showed retention of Rad51 after γ-radiation.
CONCLUSIONS: Our findings provide further understanding as to how HPV16 E7 manipulates cellular DNA damage responses that may underlie its oncogenic potential and influence the altered sensitivity to radiation seen in HPV+ HNC as compared to HPV- HNC.
PMID:25216575 | PMC:PMC4268372 | DOI:10.1016/j.radonc.2014.08.026
View details for PubMedID 25216575
-
More
-
AXL mediates resistance to cetuximab therapy Cancer research
Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, Toulany M, Gill PS, Salgia R, Kimple RJ, Wheeler DL
2014 Sep 15;74(18):5152-64. doi: 10.1158/0008-5472.CAN-14-0294. Epub 2014 Aug 18.
-
More
The EGFR antibody cetuximab is used to treat numerous cancers, but intrinsic and acquired resistance to this agent is a common clinical outcome. In this study, we show that overexpression of the oncogenic receptor tyrosine kinase AXL is sufficient to mediate acquired resistance to cetuximab in models of non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma (HNSCC), where AXL was overexpressed, activated, and tightly associated with EGFR expression in cells resistant to cetuximab (Ctx(R) cells). Using RNAi methods and novel AXL-targeting agents, we found that AXL activation stimulated cell proliferation, EGFR activation, and MAPK signaling in Ctx(R) cells. Notably, EGFR directly regulated the expression of AXL mRNA through MAPK signaling and the transcription factor c-Jun in Ctx(R) cells, creating a positive feedback loop that maintained EGFR activation by AXL. Cetuximab-sensitive parental cells were rendered resistant to cetuximab by stable overexpression of AXL or stimulation with EGFR ligands, the latter of which increased AXL activity and association with the EGFR. In tumor xenograft models, the development of resistance following prolonged treatment with cetuximab was associated with AXL hyperactivation and EGFR association. Furthermore, in an examination of patient-derived xenografts established from surgically resected HNSCCs, AXL was overexpressed and activated in tumors that displayed intrinsic resistance to cetuximab. Collectively, our results identify AXL as a key mediator of cetuximab resistance, providing a rationale for clinical evaluation of AXL-targeting drugs to treat cetuximab-resistant cancers. Cancer Res; 74(18); 5152-64. ©2014 AACR.
PMID:25136066 | PMC:PMC4167493 | DOI:10.1158/0008-5472.CAN-14-0294
View details for PubMedID 25136066
-
More
-
Wetting the whistle: neurotropic factor improves salivary function The Journal of clinical investigation
Swick A, Kimple RJ
2014 Aug;124(8):3282-4. doi: 10.1172/JCI77194. Epub 2014 Jul 18.
-
More
Xerostomia, or dry mouth, is a common side effect of head and neck radiotherapy, Sjögren syndrome, diabetes, old age, and numerous medications. In this issue of the JCI, Xiao and colleagues identified glial cell line-derived neurotrophic factor (GDNF) as a potential stimulus for salivary stem cell growth. Due to its ability to promote neuronal growth, differentiation, and survival, GDNF is currently being used in clinical trials as a treatment for Parkinson disease; therefore, the findings of Xiao and colleagues may initiate a potential treatment for the millions of patients who suffer from xerostomia each year.
PMID:25036702 | PMC:PMC4109536 | DOI:10.1172/JCI77194
View details for PubMedID 25036702
-
More
-
Influence of handling conditions on the establishment and propagation of head and neck cancer patient derived xenografts PloS one
Stein AP, Saha S, Liu CZ, Hartig GK, Lambert PF, Kimple RJ
2014 Jun 26;9(6):e100995. doi: 10.1371/journal.pone.0100995. eCollection 2014.
-
More
BACKGROUND: Patient derived xenografts (PDXs) for head and neck cancer (HNC) and other cancers represent powerful research platforms. Most groups implant patient tissue into immunodeficient mice immediately although the significance of this time interval is anecdotal. We tested the hypothesis that the time from tumor excision to implantation is crucial for PDX passaging and establishment.
METHODS: We examined whether time or storage medium affected PDX viability for passaging two established HNC PDXs (UW-SCC34, UW-SCC52). Tumors were harvested, stored in ice-cold media or saline for 0-48 hours, and implanted into new mice. Tumor growth was compared by two-way ANOVA with respect to time and storage condition. Three new HNC PDXs (UW-SCC63-65) were generated by implanting patient tissue into mice immediately (Time 0) and 24 hours after receiving tissue from the operating room.
RESULTS: Similar quantities of tumor were implanted into each mouse. At the end of the experiment, no significant difference was seen in mean tumor weight between the media and saline storage conditions for UW-SCC34 or UW-SCC52 (p = 0.650 and p = 0.177, respectively). No difference in tumor formation prevalence was seen on the basis of time from harvest to implantation (≥13 of 16 tumors grew at every time point). Histological analysis showed strong similarity to the initial tumor across all groups. Tumors developed at both Time 0 and 24 hours for UW-SCC63 and UW-SCC64.
CONCLUSIONS: We demonstrated that neither storage medium nor time from tumor excision to implantation (up to 48 hours) affected viability or histological differentiation in a subsequent passage for two HNC PDXs. Moreover, we revealed that fresh patient tissue is viable up to 24 hours post-resection. This information is important as it applies to the development and sharing of PDXs.
PMID:24967635 | PMC:PMC4072729 | DOI:10.1371/journal.pone.0100995
View details for PubMedID 24967635
-
More
Contact Information
Randall Kimple, MD, PhD
1111 Highland Avenue,3107 WIMR
Madison, WI 53705-2275