I am an Associate professor and Chair of the Department of Human Oncology. I am originally from Rockford, IL, and completed my undergraduate studies at Ripon College in Ripon, Wis. After my undergraduate work, I earned two Master’s degrees (Medical Anthropology and History of Science, Medicine, and Technology) at Oxford University as a Rhodes Scholar. I completed my MD at Harvard Medical School and my PhD at Harvard University in the Biological and Biomedical Sciences Program, where I performed thesis research in the laboratory of Prof. Andrea McClatchey.
I completed a preliminary year internship in internal medicine at the University of Hawaii and then completed my residency training in radiation oncology at the University of Wisconsin Hospitals and Clinics. Under the American Board of Radiology’s Holman Pathway, I spearheaded a collaborative research project during my residency in the labs of Prof. Paul Sondel and Prof. Paul Harari.
As a physician-scientist, my current clinical focus is on the treatment of patients with melanoma and soft tissue sarcomas. My independent translational research laboratory focuses on the mechanisms whereby radiation may enhance the response to immunotherapies. I serve as program director for the University of Wisconsin Bentson Research Fellowship and am an active member of ASTRO, ASCO, RSNA, ABS, ACR, AACR, Radiation Research Society, the Society for Immunotherapy in Cancer (SITC) and the NCCN Guidelines expert panel on soft tissue sarcomas.
Education
Resident, University of Wisconsin–Madison, Radiation Oncology (2016)
Intern, University of Hawaii, Internal Medicine (2012)
MD, Harvard Medical School, Medicine (2011)
PhD, Harvard University, Biological and Biomedical Sciences (2011)
MSc, Oxford University, History of Science, Medicine and Technology (2004)
MSc, Oxford University, Medical Anthropology (2003)
BA, Ripon College, Chemistry and Biology (2002)
Academic Appointments
Associate Professor, Human Oncology (2021)
Vice Chair, Human Oncology (2018)
Program Director, Bentson Translational Research Fellowship, Human Oncology (2017)
Assistant Professor, Human Oncology (2016)
Selected Honors and Awards
The Ride Scholar Award, University of Wisconsin (2016)
Outstanding Young Investigator, Immuno-Oncology Young Investigator’s Forum (2015)
Resident Seed Grant Recipient, American Society for Radiation Oncology (ASTRO) (2014)
Research Resident Grant Recipient, Radiological Society of North America (RSNA) (2013)
Intern of the Year, University of Hawaii Internal Medicine Residency Program (2012)
Albert Schweitzer Fellow (Boston) (2005)
Rhodes Scholar (Wisconsin & Wadham) (2002)
Senator Barry M. Goldwater Scholarship (2001)
Wisconsin Independent College Foundation Rath Scholarship (2001)
U.S. Department of Energy, Energy Research Undergraduate Fellowship (2000)
Boards, Advisory Committees and Professional Organizations
Big Ten Cancer Research Consortium Head and Neck Working Group (2017–pres.)
American College of Radiology (ACR), Radiation Oncology Commission Young Physician Section Chair (2017–pres.)
American College of Radiology (ACR), Journal Advisor, Radiobiology Expert Editor (2017–pres.)
American Brachytherapy Society Member (2016–pres.)
American Brachytherapy Society International Committee Task Group Co-Chair (2016–pres.)
National Cancer Center Network (NCCN) Member (2016–pres.)
National Cancer Center Network (NCCN) Soft Tissue Sarcoma Expert Panel Member (2016–pres.)
University of Wisconsin Institute for Clinical and Translational Research (2016–pres.)
American Society for Radiation Oncology (ASTRO), State Captain (2016–pres.)
ASTRO Annual Meeting Scientific/Education Programing Abstract Reviewer (2016–pres.)
Radiation Research Society, Sponsored Faculty Member (2016–pres.)
American Academy of Cancer Research (AACR) Member (2016–pres.)
ASTRO Annual Meeting Scientific Committee Member (2015–pres.)
ASTRO Community of Radiation Oncology Physician Scientists (CROPS) (2015–pres.)
ASTRO International Education Subcommittee Member (2015–pres.)
American College of Radiology (ACR) Journal Advisor, ARRO Guest Editor (2015–pres.)
Society for Immunotherapy in Cancer (SITC) Early Career Scientist Committee Member (2015–2016)
American College of Radiology (ACR) Resident and Fellow Section International Outreach Subcommittee Member (2015–2016)
Association of Residents in Radiation Oncology (ARRO) Executive Committee (Elected) (2014–2016)
American Society for Radiation Oncology (ASTRO) Member (2012–pres.)
Association of Residents in Radiation Oncology (ARRO) Co-chair, Global Health Subcommittee (2014–2016)
Association of Residents in Radiation Oncology (ARRO) Director, Mutual Mentorship Program (2014–2016)
Association of Residents in Radiation Oncology (ARRO) Founder and Director, Global Health Rotation Initiative (2014–2016)
Society for Immunotherapy in Cancer (SITC) Member (2013–pres.)
American Society for Clinical Oncology (ASCO) Member (2012–pres.)
Radiological Society of North America (RSNA) Member (2012–pres.)
American College of Radiology (ACR) Member (2012–pres.)
Research Focus
Radiation therapy, Immunotherapy, Melanoma, Sarcoma, Head and Neck Cancer
Dr. Zachary Morris treats patients with melanoma and soft tissue sarcomas. His independent translational research laboratory focuses on the mechanisms whereby radiation may enhance the response to immunotherapies. He also serves as vice chair of the department and program director for the University of Wisconsin Bentson Translational Research Fellowship.
Radiation may augment the local and systemic anti-tumor immune response to cancer immunotherapies.
In the Morris Lab, we are focused on using preclinical and translational research approaches to study the mechanisms whereby radiation may impact the anti-tumor response to immunotherapies. Our primary objective is to determine whether and how radiation may optimally be employed to simultaneously modulate the tumor immune microenvironment and to increase the susceptibility of tumor cells to immune response. We seek to test these approaches in early phase clinical studies where they may be further refined with the ultimate aim of improving survival and achieving cures in patients with metastatic cancers.
In Situ Tumor Vaccination
In situ tumor vaccination is a therapeutic strategy that seeks to convert a patient’s own tumor into a nidus for enhanced presentation of tumor-specific antigens in a way that will stimulate and diversify an anti-tumor T cell response. Radiation therapy elicits an anti-tumor effect through induction of DNA damage in tumor cells, yet it has long been recognized that host immune capability and tumor immune susceptibility modulate the sensitivity of a tumor to radiation. The mechanisms by which local radiation may interact with the immune system include release of tumor-specific antigens, phenotypic changes in tumor cell expression of immune susceptibility markers and local eradication of suppressive immune cell lineages. By modulating tumor immune tolerance and functional immunogenicity at a targeted site, radiation may serve as a method of in situ tumor vaccination. Multiple preclinical studies demonstrate that random tumor-specific protein mutations are among the most immunogenic tumor antigens recognized by T cells. By rendering such antigens accessible to immune recognition, radiation may augment the local and systemic anti-tumor response to immunotherapy. If proven effective, such combinations might transform RT from a predominantly loco-regional treatment to a critical component of systemic therapy.
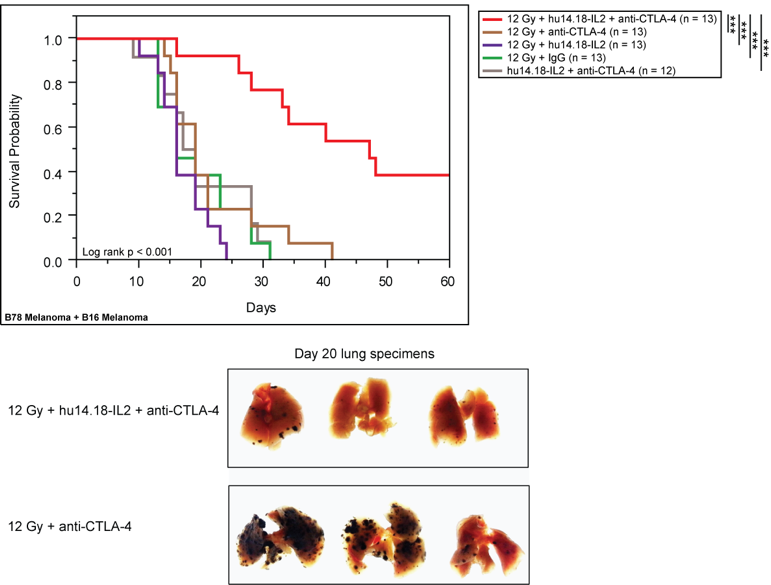
Relevant publications
Morris ZS, Guy EI, Francis DM, Gressett MM, Werner LR, Carmichael LL, Yang LL, Armstrong EA, Huang S, Navid JAF, Gillies SD, Hank JA, Rakhmilevich AL, Harari PM, Sondel PM. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Research. 2016 Jul 1;76(13):3929-41. PMID: 27197149.
Funding
- NIH Director’s Early Independence Award (DP5) 9/2017– 8/2022. Combining radiation and tumor-specific antibody therapies to elicit in situ vaccination. Role: Principal Investigator
- UWCCC Tumor Immunology/Cancer Immunotherapy Pilot Award 7/2017 – 6/2018. In situ vaccination to redress clinical challenges in the treatment of metastatic melanoma. Role: Principal Investigator (Morris, Kuo)
Effects of Radiation on Tumor Cell Immune Susceptibility
Prior studies have consistently demonstrated phenotypic upregulation of FAS and MHC-I following radiation therapy, and recent studies have suggested mechanisms whereby radiation may influence tumor expression of the checkpoint ligand, PD-L1. The time course, potentially shared underlying mechanisms and the possibility of a broader impact of radiation on expression of other phenotypic markers of tumor immune susceptibility remain to be clarified. Given the potential capacity of radiation to synergize with immunotherapies, it is vital to understand not only how radiation may affect tumor cell susceptibility to immune response but also to define the time course and mechanism of such effects.
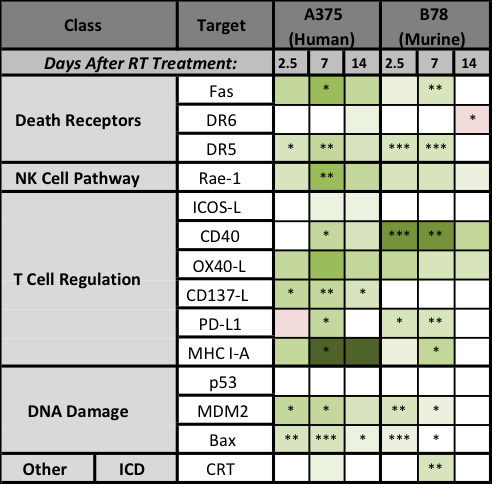
Relevant publications
Werner LR, Kler JS, Gressett MM, Riegert M, Werner LK, Heinze CM, Kern JG, Abbariki M, Erbe AK, Patel RB, Sriramaneni RN, Harari PM, Morris ZS. Transcriptional-mediated effects of radiation on the expression of immune susceptibility markers in melanoma. Radiother Oncol. 2017 Sep 8. pii: S0167-8140(17)32526-4. PMID: 28893414.
Funding
- NIH Director’s Early Independence Award (DP5) 9/2017–8/2022. Combining radiation and tumor-specific antibody therapies to elicit in situ vaccination. Role: Principal Investigator
Development of new models and methods for testing novel combinations of radiation with immunotherapy
In a series of “next generation” studies, we are developing novel tumor models that will enable us to better test the efficacy of combinations of radiation with immunotherapies. At the same time, we are also exploring new methods for evaluating the mechanisms of interaction between these treatment modalities. In addition, we are actively exploring multiple unique approaches to optimizing this cooperative interaction through novel combinations of distinct radiotherapy modalities with diverse immunotherapies.
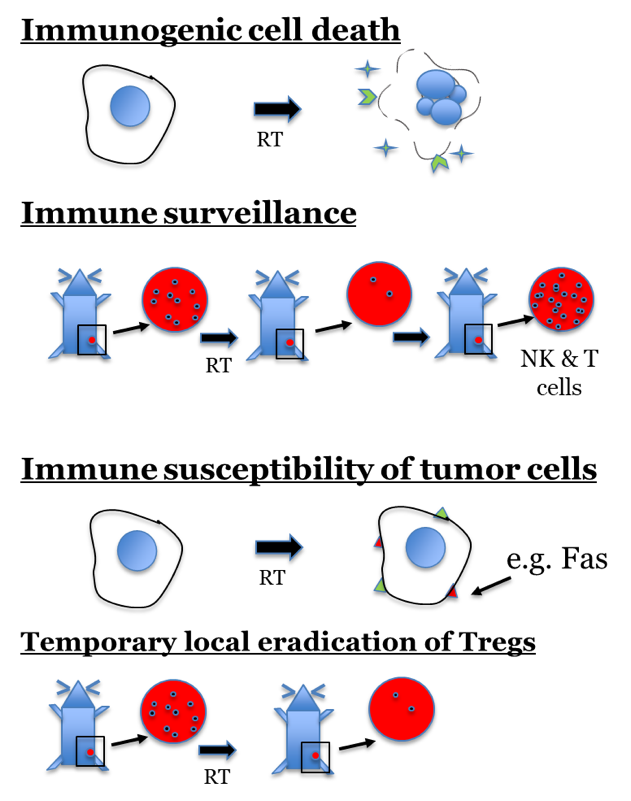
Funding
- Wisconsin Alumni Research Foundation 20/20 Award 10/2016–9/2018. Combining molecular targeted radiation with antitumor mAb and IL2 to create a potent in situ cancer vaccine. Role: Co-Principal Investigator (Sondel, Morris, Weichert, Bednarz, Otto)
- The Ride Scholars 1/2017–12/2017. Pilot investigation of novel combinations of molecular targeted immunotherapy with radiation. Role: Principal Investigator
- UWCCC Tumor Microenvironment Pilot Award 7/2017– 6/2018. Development of patient-derived xenografts in humanized mice. Role: Co-Principal Investigator (Kimple, Morris)
- RSNA Fellows Research Award 7/2017–6/2018. Utilization of Radiotherapy to Enhance the Efficacy of Systemic Dual Checkpoint Inhibition in Preclinical Metastatic Cancer Models. Role: Scientific Mentor for Ravi Patel
- UWCCC Tumor Immunology/Cancer Immunotherapy Pilot Award 7/2017–6/2018. In situ vaccination to redress clinical challenges in the treatment of metastatic melanoma. Role: Principal Investigator (Morris, Kuo)
- UW H&N SPORE Career Enhancement Program Award 7/2017–6/2018. Development of syngeneic murine head and neck squamous cell carcinoma tumor models for testing in situ tumor vaccination therapeutic approaches. Role: Principal Investigator
Early phase clinical trial development and correlative studies
As a physician-scientist, I help lead early phase clinical research efforts aimed at translating findings from our preclinical research to the clinic in order to improve treatment of cancer patients. We are currently advancing early phase clinical studies involving a variety of disease sites with the goal of improving clinical outcomes and cure rates for patients with cancer. Correlative biomarker studies and tissue samples from these clinical studies will be instrumental in allowing us to test the translational relevance of our preclinical findings, and this should enable us to refine our approaches to achieve greater clinical effect.
Locations:
University of Wisconsin Hospitals and Clinics
EARLY PHASE CLINICAL TRIAL DEVELOPMENT AND CORRELATIVE STUDIES
As a physician-scientist, I help lead early phase clinical research efforts aimed at translating findings from our preclinical research to the clinic in order to improve treatment of cancer patients. We are currently advancing early phase clinical studies involving a variety of different disease sites with the goal of improving clinical outcomes and cure rates for patients with cancer. Correlative biomarker studies and tissue samples from these clinical studies will be instrumental in allowing us to test the translational relevance of our preclinical findings and this should enable us to refine our approaches to achieve greater clinical effect.
-
Large-scale discovery of chromatin dysregulation induced by oncofusions and other protein-coding variants Nature biotechnology
Frenkel M, Corban JE, Hujoel LA, Morris Z, Raman S
2024 Jul 24. doi: 10.1038/s41587-024-02347-4. Online ahead of print.
-
More
Population-scale databases have expanded to millions of protein-coding variants, yet insight into their mechanistic consequences has lagged. Here we present PROD-ATAC, a high-throughput method for discovering the effects of protein-coding variants on chromatin regulation. A pooled variant library is expressed in a disease-agnostic cell line, and single-cell assay for transposase-accessible chromatin resolves each variant's effect on the chromatin landscape. Using PROD-ATAC, we characterized the effects of more than 100 oncofusions (cancer-causing chimeric proteins) and controls and revealed that chromatin remodeling is common to fusions spanning an enormous range of fusion frequencies. Furthermore, fusion-induced dysregulation can be context agnostic, as observed mechanisms often overlapped with cancer and cell-type-specific prior knowledge. We also showed that gain-of-function activity is common among oncofusions. This work begins to outline a global map of fusion-induced chromatin alterations. We suggest that there might be convergent mechanisms among disparate oncofusions and shared modes of dysregulation among fusions present in tumors at different frequencies. PROD-ATAC is generalizable to any set of protein-coding variants.
PMID:39048711 | DOI:10.1038/s41587-024-02347-4
View details for PubMedID 39048711
-
More
-
The Effects of Radiation Dose Heterogeneity on the Tumor Microenvironment and Anti-Tumor Immunity Seminars in radiation oncology
Takashima ME, Berg TJ, Morris ZS
2024 Jul;34(3):262-271. doi: 10.1016/j.semradonc.2024.04.004.
-
More
Radiotherapy elicits dose- and lineage-dependent effects on immune cell survival, migration, activation, and proliferation in targeted tumor microenvironments. Radiation also stimulates phenotypic changes that modulate the immune susceptibility of tumor cells. This has raised interest in using radiotherapy to promote greater response to immunotherapies. To clarify the potential of such combinations, it is critical to understand how best to administer radiation therapy to achieve activation of desired immunologic mechanisms. In considering the multifaceted process of priming and propagating anti-tumor immune response, radiation dose heterogeneity emerges as a potential means for simultaneously engaging diverse dose-dependent effects in a single tumor environment. Recent work in spatially fractionated external beam radiation therapy demonstrates the expansive immune responses achievable when a range of high to low dose radiation is delivered in a tumor. Brachytherapy and radiopharmaceutical therapies deliver inherently heterogeneous distributions of radiation that may contribute to immunogenicity. This review evaluates the interplay of radiation dose and anti-tumor immune response and explores emerging methodological approaches for investigating the effects of heterogeneous dose distribution on immune responses.
PMID:38880534 | DOI:10.1016/j.semradonc.2024.04.004
View details for PubMedID 38880534
-
More
-
Administration of intratumoral GD2-directed interleukin-2 immunocytokine and local radiation therapy to activate immune rejection of spontaneous canine melanoma Melanoma research
Albertini MR, Zuleger CL, Ranheim EA, Shiyanbola O, Sondel PM, Morris ZS, Eickhoff J, Newton MA, Ong IM, Schwartz RW, Hayim R, Kurzman ID, Turek M, Vail DM
2024 Aug 1;34(4):307-318. doi: 10.1097/CMR.0000000000000975. Epub 2024 May 20.
-
More
Canine malignant melanoma provides a clinically relevant, large animal parallel patient population to study the GD2-reactive hu14.18-IL-2 immunocytokine as it is similar to human melanoma and expresses GD2. The objectives of this study were to evaluate safety, radiation fractionation, and identify informative biomarkers of an in-situ tumor vaccine involving local radiation therapy plus intratumoral-immunocytokine in melanoma tumor-bearing dogs. Twelve dogs (six dogs/arm) with locally advanced or metastatic melanoma were randomized to receive a single 8 Gy fraction (arm A) or three 8 Gy fractions over 1 week (arm B) to the primary site and regional lymph nodes (when clinically involved) with the single or last fraction 5 days before intratumoral-immunocytokine at 12 mg/m 2 on 3 consecutive days. Serial tumor biopsies were obtained. All 12 dogs completed protocol treatment, and none experienced significant or unexpected adverse events. Evidence of antitumor activity includes one dog with a complete response at day 60, one dog with a partial response at day 60, and four dogs with mixed responses. Histology of serial biopsies shows a variably timed increase in intratumoral lymphocytic inflammation in some dogs. Canine NanoString analyses of serial biopsies identified changes in gene signatures of innate and adaptive cell types versus baseline. There were no significant differences in NanoString results between arm A and arm B. We conclude that intratumoral-immunocytokine in combination with local radiation therapy in canine melanoma is well tolerated and has antitumor activity with the potential to inform clinical development in melanoma patients.
PMID:38768442 | DOI:10.1097/CMR.0000000000000975
View details for PubMedID 38768442
-
More
-
Myeloid-derived suppressor cells attenuate the antitumor efficacy of radiopharmaceutical therapy using <sup>90</sup>Y-NM600 in combination with androgen deprivation therapy in murine prostate tumors Journal for immunotherapy of cancer
Muralidhar A, Hernandez R, Morris ZS, Rojas HC, Idrissou MB, Weichert JP, McNeel DG
2024 Apr 24;12(4):e008760. doi: 10.1136/jitc-2023-008760.
-
More
RATIONALE: Androgen deprivation therapy (ADT) is pivotal in treating recurrent prostate cancer and is often combined with external beam radiation therapy (EBRT) for localized disease. However, for metastatic castration-resistant prostate cancer, EBRT is typically only used in the palliative setting, because of the inability to radiate all sites of disease. Systemic radiation treatments that preferentially irradiate cancer cells, known as radiopharmaceutical therapy or targeted radionuclide therapy (TRT), have demonstrable benefits for treating metastatic prostate cancer. Here, we explored the use of a novel TRT, 90Y-NM600, specifically in combination with ADT, in murine prostate tumor models.
METHODS: 6-week-old male FVB mice were implanted subcutaneously with Myc-CaP tumor cells and given a single intravenous injection of 90Y-NM600, in combination with ADT (degarelix). The combination and sequence of administration were evaluated for effect on tumor growth and infiltrating immune populations were analyzed by flow cytometry. Sera were assessed to determine treatment effects on cytokine profiles.
RESULTS: ADT delivered prior to TRT (ADT→TRT) resulted in significantly greater antitumor response and overall survival than if delivered after TRT (TRT→ADT). Studies conducted in immunodeficient NRG mice failed to show a difference in treatment sequence, suggesting an immunological mechanism. Myeloid-derived suppressor cells (MDSCs) significantly accumulated in tumors following TRT→ADT treatment and retained immune suppressive function. However, CD4+ and CD8+ T cells with an activated and memory phenotype were more prevalent in the ADT→TRT group. Depletion of Gr1+MDSCs led to greater antitumor response following either treatment sequence. Chemotaxis assays suggested that tumor cells secreted chemokines that recruited MDSCs, notably CXCL1 and CXCL2. The use of a selective CXCR2 antagonist, reparixin, further improved antitumor responses and overall survival when used in tumor-bearing mice treated with TRT→ADT.
CONCLUSION: The combination of ADT and TRT improved antitumor responses in murine models of prostate cancer, however, this was dependent on the order of administration. This was found to be associated with one treatment sequence leading to an increase in infiltrating MDSCs. Combining treatment with a CXCR2 antagonist improved the antitumor effect of this combination, suggesting a possible approach for treating advanced human prostate cancer.
PMID:38663936 | PMC:PMC11043705 | DOI:10.1136/jitc-2023-008760
View details for PubMedID 38663936
-
More
-
Functionality of bone marrow mesenchymal stromal cells derived from head and neck cancer patients - A FDA-IND enabling study regarding MSC-based treatments for radiation-induced xerostomia Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Blitzer GC, Paz C, Glassey A, Ganz OR, Giri J, Pennati A, Meyers RO, Bates AM, Nickel KP, Weiss M, Morris ZS, Mattison RJ, McDowell KA, Croxford E, Chappell RJ, Glazer TA, Rogus-Pulia NM, Galipeau J, Kimple RJ
2024 Mar;192:110093. doi: 10.1016/j.radonc.2024.110093. Epub 2024 Jan 13.
-
More
PURPOSE: Salivary dysfunction is a significant side effect of radiation therapy for head and neck cancer (HNC). Preliminary data suggests that mesenchymal stromal cells (MSCs) can improve salivary function. Whether MSCs from HNC patients who have completed chemoradiation are functionally similar to those from healthy patients is unknown. We performed a pilot clinical study to determine whether bone marrow-derived MSCs [MSC(M)] from HNC patients could be used for the treatment of RT-induced salivary dysfunction.
METHODS: An IRB-approved pilot clinical study was undertaken on HNC patients with xerostomia who had completed treatment two or more years prior. Patients underwent iliac crest bone marrow aspirate and MSC(M) were isolated and cultured. Culture-expanded MSC(M) were stimulated with IFNγ and cryopreserved prior to reanimation and profiling for functional markers by flow cytometry and ELISA. MSC(M) were additionally injected into mice with radiation-induced xerostomia and the changes in salivary gland histology and salivary production were examined.
RESULTS: A total of six subjects were enrolled. MSC(M) from all subjects were culture expanded to > 20 million cells in a median of 15.5 days (range 8-20 days). Flow cytometry confirmed that cultured cells from HNC patients were MSC(M). Functional flow cytometry demonstrated that these IFNγ-stimulated MSC(M) acquired an immunosuppressive phenotype. IFNγ-stimulated MSC(M) from HNC patients were found to express GDNF, WNT1, and R-spondin 1 as well as pro-angiogenesis and immunomodulatory cytokines. In mice, IFNγ-stimulated MSC(M) injection after radiation decreased the loss of acinar cells, decreased the formation of fibrosis, and increased salivary production.
CONCLUSIONS: MSC (M) from previously treated HNC patients can be expanded for auto-transplantation and are functionally active. Furthermore IFNγ-stimulated MSC(M) express proteins implicated in salivary gland regeneration. This study provides preliminary data supporting the feasibility of using autologous MSC(M) from HNC patients to treat RT-induced salivary dysfunction.
PMID:38224919 | PMC:PMC10922976 | DOI:10.1016/j.radonc.2024.110093
View details for PubMedID 38224919
-
More
-
Comparative Study of the Effect of Radiation Delivered by Lutetium-177 or Actinium-225 on Anti-GD2 Chimeric Antigen Receptor T Cell Viability and Functions Cancers
Sodji QH, Forsberg MH, Cappabianca D, Kerr CP, Sarko L, Shea A, Adam DP, Eickhoff JC, Ong IM, Hernandez R, Weichert J, Bednarz BP, Saha K, Sondel PM, Capitini CM, Morris ZS
2023 Dec 30;16(1):191. doi: 10.3390/cancers16010191.
-
More
Chimeric antigen receptor (CAR) T cells have been relatively ineffective against solid tumors. Low-dose radiation which can be delivered to multiple sites of metastases by targeted radionuclide therapy (TRT) can elicit immunostimulatory effects. However, TRT has never been combined with CAR T cells against solid tumors in a clinical setting. This study investigated the effects of radiation delivered by Lutetium-177 (177Lu) and Actinium-225 (225Ac) on the viability and effector function of CAR T cells in vitro to evaluate the feasibility of such therapeutic combinations. After the irradiation of anti-GD2 CAR T cells with various doses of radiation delivered by 177Lu or 225Ac, their viability and cytotoxic activity against GD2-expressing human CHLA-20 neuroblastoma and melanoma M21 cells were determined by flow cytometry. The expression of the exhaustion marker PD-1, activation marker CD69 and the activating receptor NKG2D was measured on the irradiated anti-GD2 CAR T cells. Both 177Lu and 225Ac displayed a dose-dependent toxicity on anti-GD2 CAR T cells. However, radiation enhanced the cytotoxic activity of these CAR T cells against CHLA-20 and M21 irrespective of the dose tested and the type of radionuclide. No significant changes in the expression of PD-1, CD69 and NKG2D was noted on the CAR T cells following irradiation. Given a lower CAR T cell viability at equal doses and an enhancement of cytotoxic activity irrespective of the radionuclide type, 177Lu-based TRT may be preferred over 225Ac-based TRT when evaluating a potential synergism between these therapies in vivo against solid tumors.
PMID:38201618 | PMC:PMC10778389 | DOI:10.3390/cancers16010191
View details for PubMedID 38201618
-
More
-
ACR-ACNM-ARS-ASTRO-SNMMI Practice Parameter for the Performance of Therapy With Radiopharmaceuticals American journal of clinical oncology
Wallner PE, Yoo DC, Calais J, Escorcia FE, Aparici CM, Michalski J, Morris M, Morris ZS, Pryma D, Rabatic BM, Sharma N, Vapiwala N, Ghesani MV, Subramaniam RM, Small W, Schechter NR
2024 Apr 1;47(4):169-176. doi: 10.1097/COC.0000000000001072. Epub 2023 Dec 22.
-
More
OBJECTIVES: This practice parameter was revised collaboratively by the American College of Radiology (ACR), the American College of Nuclear Medicine, the American Radium Society, the American Society for Radiation Oncology, and the Society of Nuclear Medicine and Molecular Imaging. The document is intended to serve as a resource for appropriately trained and licensed physicians who perform therapeutic procedures with unsealed sources, referred to in the document using the more inclusive terminology of radiopharmaceuticals, for which a written directive is required for authorized users under NRC 10 CFR 35.300.
METHODS: This practice parameter was developed according to the process described under the heading The Process for Developing ACR Practice Parameters and Technical Standards on the ACR website ( https://www.acr.org/Clinical-Resources/Practice-Parameters-and-Technical-Standards ) by the Committee on Practice Parameters-Radiation Oncology of the ACR Commission on Radiation Oncology in collaboration with the American Radium Society.
RESULTS: This practice parameter addresses the overall role of the applicable physician-authorized user, Qualified Medical Physicist, and other specialized personnel involved in the delivery of radiopharmaceutical therapy. Therapeutic radiopharmaceuticals include those administered as elemental radioactive isotopes (radionuclides) or the radioactive element incorporated into a targeting molecule (ligand) by one or more chemical bonds. This document provides guidance regarding general principles of radionuclide therapies and indications of various alpha, beta, gamma, and mixed emission agents with references to several recent practice parameters on new and commonly performed radiopharmaceutical therapies.
CONCLUSION: This document addresses clinical circumstances, elements of available agents, and the qualifications and responsibilities of various members of the radiation care team, specifications of consultation and other clinical documentation, post-therapy follow-up, radiation safety precautions, elements of quality control and improvement programs, infection control, and patient education to ensure optimal patient care and safety when utilizing radiopharmaceuticals.
PMID:38131352 | DOI:10.1097/COC.0000000000001072
View details for PubMedID 38131352
-
More
-
NK cells propagate T cell immunity following in situ tumor vaccination Cell reports
Jin WJ, Jagodinsky JC, Vera JM, Clark PA, Zuleger CL, Erbe AK, Ong IM, Le T, Tetreault K, Berg T, Rakhmilevich AL, Kim K, Newton MA, Albertini MR, Sondel PM, Morris ZS
2023 Dec 26;42(12):113556. doi: 10.1016/j.celrep.2023.113556. Epub 2023 Dec 13.
-
More
We report an in situ vaccination, adaptable to nearly any type of cancer, that combines radiotherapy targeting one tumor and intratumoral injection of this site with tumor-specific antibody and interleukin-2 (IL-2; 3xTx). In a phase I clinical trial, administration of 3xTx (with an immunocytokine fusion of tumor-specific antibody and IL-2, hu14.18-IL2) to subjects with metastatic melanoma increases peripheral CD8+ T cell effector polyfunctionality. This suggests the potential for 3xTx to promote antitumor immunity against metastatic tumors. In poorly immunogenic syngeneic murine melanoma or head and neck carcinoma models, 3xTx stimulates CD8+ T cell-mediated antitumor responses at targeted and non-targeted tumors. During 3xTx treatment, natural killer (NK) cells promote CTLA4+ regulatory T cell (Treg) apoptosis in non-targeted tumors. This is dependent on NK cell expression of CD86, which is upregulated downstream of KLRK1. NK cell depletion increases Treg infiltration, diminishing CD8+ T cell-dependent antitumor response. These findings demonstrate that NK cells sustain and propagate CD8+ T cell immunity following 3xTx.
PMID:38096050 | PMC:PMC10843551 | DOI:10.1016/j.celrep.2023.113556
View details for PubMedID 38096050
-
More
-
Antibody landscape of C57BL/6 mice cured of B78 melanoma via a combined radiation and immunocytokine immunotherapy regimen Frontiers in immunology
Hoefges A, McIlwain SJ, Erbe AK, Mathers N, Xu A, Melby D, Tetreault K, Le T, Kim K, Pinapati RS, Garcia BH, Patel J, Heck M, Feils AS, Tsarovsky N, Hank JA, Morris ZS, Ong IM, Sondel PM
2023 Nov 23;14:1221155. doi: 10.3389/fimmu.2023.1221155. eCollection 2023.
-
More
Sera of immune mice that were previously cured of their melanoma through a combined radiation and immunocytokine immunotherapy regimen consisting of 12 Gy of external beam radiation and the intratumoral administration of an immunocytokine (anti-GD2 mAb coupled to IL-2) with long-term immunological memory showed strong antibody-binding against melanoma tumor cell lines via flow cytometric analysis. Using a high-density whole-proteome peptide array (of 6.090.593 unique peptides), we assessed potential protein-targets for antibodies found in immune sera. Sera from 6 of these cured mice were analyzed with this high-density, whole-proteome peptide array to determine specific antibody-binding sites and their linear peptide sequence. We identified thousands of peptides that were targeted by these 6 mice and exhibited strong antibody binding only by immune (after successful cure and rechallenge), not naïve (before tumor implantation) sera and developed a robust method to detect these differentially targeted peptides. Confirmatory studies were done to validate these results using 2 separate systems, a peptide ELISA and a smaller scale peptide array utilizing a slightly different technology. To the best of our knowledge, this is the first study of the full set of germline encoded linear peptide-based proteome epitopes that are recognized by immune sera from mice cured of cancer via radio-immunotherapy. We furthermore found that although the generation of B-cell repertoire in immune development is vastly variable, and numerous epitopes are identified uniquely by immune serum from each of these 6 immune mice evaluated, there are still several epitopes and proteins that are commonly recognized by at least half of the mice studied. This suggests that every mouse has a unique set of antibodies produced in response to the curative therapy, creating an individual "fingerprint." Additionally, certain epitopes and proteins stand out as more immunogenic, as they are recognized by multiple mice in the immune group.
PMID:38077403 | PMC:PMC10701281 | DOI:10.3389/fimmu.2023.1221155
View details for PubMedID 38077403
-
More
-
Combining Dual Checkpoint Immunotherapy with Ablative Radiation to All Sites of Oligometastatic Non-Small Cell Lung Cancer: Toxicity and Efficacy Results of a Phase 1b Trial International journal of radiation oncology, biology, physics
Bassetti MF, Morris BA, Sethakorn N, Lang JM, Schehr JL, Zhao SG, Morris ZS, Buehler D, Eickhoff JC, Harari PM, Traynor AM, Campbell TC, Baschnagel AM, Leal TA
2024 Apr 1;118(5):1481-1489. doi: 10.1016/j.ijrobp.2023.11.040. Epub 2023 Dec 8.
-
More
PURPOSE: Ablative local treatment of all radiographically detected metastatic sites in patients with oligometastatic non-small cell lung cancer (NSCLC) increases progression-free survival (PFS) and overall survival (OS). Prior studies demonstrated the safety of combining stereotactic body radiation therapy (SBRT) with single-agent immunotherapy. We investigated the safety of combining SBRT to all metastatic tumor sites with dual checkpoint, anticytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), and anti-programmed cell death ligand 1 (anti-PD-L1) immunotherapy for patients with oligometastatic NSCLC.
METHODS AND MATERIALS: We conducted a phase 1b clinical trial in patients with oligometastatic NSCLC with up to 6 sites of extracranial metastatic disease. All sites of disease were treated with SBRT to a dose of 30 to 50 Gy in 5 fractions. Dual checkpoint immunotherapy was started 7 days after completion of radiation using anti-CTLA-4 (tremelimumab) and anti-PD-L1 (durvalumab) immunotherapy for a total of 4 cycles followed by durvalumab alone until progression or toxicity.
RESULTS: Of the 17 patients enrolled in this study, 15 patients received at least 1 dose of combination immunotherapy per protocol. The study was closed early (17 of planned 21 patients) due to slow accrual during the COVID-19 pandemic. Grade 3+ treatment-related adverse events were observed in 6 patients (40%), of which only one was possibly related to the addition of SBRT to immunotherapy. Median PFS was 42 months and median OS has not yet been reached.
CONCLUSIONS: Delivering ablative SBRT to all sites of metastatic disease in combination with dual checkpoint immunotherapy did not result in excessive rates of toxicity compared with historical studies of dual checkpoint immunotherapy alone. Although the study was not powered for treatment efficacy results, durable PFS and OS results suggest potential therapeutic benefit compared with immunotherapy or radiation alone in this patient population.
PMID:38072321 | PMC:PMC10947887 | DOI:10.1016/j.ijrobp.2023.11.040
View details for PubMedID 38072321
-
More
-
Discovering chromatin dysregulation induced by protein-coding perturbations at scale bioRxiv : the preprint server for biology
Frenkel M, Hujoel LA, Morris Z, Raman S
2023 Sep 21:2023.09.20.555752. doi: 10.1101/2023.09.20.555752.
-
More
Although population-scale databases have expanded to millions of protein-coding variants, insight into variant mechanisms has not kept pace. We present PROD-ATAC, a high-throughput method for discovering the effects of protein-coding variants on chromatin. A pooled library of variants is expressed in a disease-agnostic cell line, and single-cell ATAC resolves each variant's effect on chromatin. Using PROD-ATAC, we characterized the effects of >100 oncofusions (a class of cancer-causing chimeric proteins) and controls and revealed that pioneer activity is a common feature of fusions spanning an enormous range of fusion frequencies. Further, fusion-induced dysregulation can be context-agnostic as observed mechanisms often overlapped with cancer and cell-type specific prior knowledge. We also showed that gain-of-function pioneering is common among oncofusions. This work provides a global view of fusion-induced chromatin. We uncovered convergent mechanisms among disparate oncofusions and shared modes of dysregulation across different cancers. PROD-ATAC is generalizable to any set of protein-coding variants.
PMID:37781603 | PMC:PMC10541138 | DOI:10.1101/2023.09.20.555752
View details for PubMedID 37781603
-
More
-
Cyclophosphamide augments the efficacy of <em>in situ</em> vaccination in a mouse melanoma model Frontiers in oncology
Tsarovsky N, Felder M, Heck M, Slowinski J, Rasmussen K, VandenHeuvel S, Zaborek J, Morris ZS, Erbe AK, Sondel PM, Rakhmilevich AL
2023 Sep 6;13:1200436. doi: 10.3389/fonc.2023.1200436. eCollection 2023.
-
More
INTRODUCTION: We have previously shown that an intratumoral (IT) injection of the hu14.18-IL2 immunocytokine (IC), an anti-GD2 antibody linked to interleukin 2, can serve as an in situ vaccine and synergize with local radiotherapy (RT) to induce T cell-mediated antitumor effects. We hypothesized that cyclophosphamide (CY), a chemotherapeutic agent capable of depleting T regulatory cells (Tregs), would augment in situ vaccination. GD2+ B78 mouse melanoma cells were injected intradermally in syngeneic C57BL/6 mice.
METHODS: Treatments with RT (12Gy) and/or CY (100 mg/kg i.p.) started when tumors reached 100-300 mm3 (day 0 of treatment), followed by five daily injections of IT-IC (25 mcg) on days 5-9. Tumor growth and survival were followed. In addition, tumors were analyzed by flow cytometry.
RESULTS: Similar to RT, CY enhanced the antitumor effect of IC. The strongest antitumor effect was achieved when CY, RT and IC were combined, as compared to combinations of IC+RT or IC+CY. Flow cytometric analyses showed that the combined treatment with CY, RT and IC decreased Tregs and increased the ratio of CD8+ cells/Tregs within the tumors. Moreover, in mice bearing two separate tumors, the combination of RT and IT-IC delivered to one tumor, together with systemic CY, led to a systemic antitumor effect detected as shrinkage of the tumor not treated directly with RT and IT-IC. Cured mice developed immunological memory as they were able to reject B78 tumor rechallenge.
CONCLUSION: Taken together, these preclinical results show that CY can augment the antitumor efficacy of IT- IC, given alone or in combination with local RT, suggesting potential benefit in clinical testing of these combinations.
PMID:37746303 | PMC:PMC10516537 | DOI:10.3389/fonc.2023.1200436
View details for PubMedID 37746303
-
More
-
ATM inhibition augments type I interferon response and antitumor T-cell immunity when combined with radiation therapy in murine tumor models Journal for immunotherapy of cancer
Jin WJ, Zangl LM, Hyun M, Massoud E, Schroeder K, Alexandridis RA, Morris ZS
2023 Sep;11(9):e007474. doi: 10.1136/jitc-2023-007474.
-
More
BACKGROUND: Radiation therapy (RT) elicits DNA double-strand breaks, resulting in tumor cytotoxicity and a type I interferon (IFN) response via stimulator of interferon genes (STING) activation. We investigated whether combining RT with an ataxia-telangiectasia mutated inhibitor promoted these effects and amplified tumor immunity.
METHODS: Mice-bearing syngeneic flank tumors (MOC2 head and neck squamous cell carcinoma or B78 melanoma) were treated with tumor-directed RT and oral administration of AZD0156. Specific immune cell depletion, type 1 interferon receptor 1 knock-out mice (IFNAR1-KO), and STING-deficient tumor cells were used to investigate tumor-immune crosstalk following RT and AZD0156 treatment.
RESULTS: Combining RT and AZD0156 reduced tumor growth compared with RT or AZD0156 alone in mice bearing MOC2 or B78 tumors. Low-dose AZD0156 (1-100 nM) alone did not affect tumor cell proliferation but suppressed tumor cell clonogenicity in combination with RT. Low-dose AZD0156 with RT synergistically increased IFN-β, major histocompatibility complex (MHC)-I, and programmed death-ligand 1 (PD-L1) expression in tumor cells. In contrast to wild-type mice, IFNAR1-KO mice showed reduced CD8+T cell tumor infiltration and poor survival following RT+AZD0156 treatment. CD8+T cell depletion reduced antitumor response during RT+AZD0156 treatment. STING-deficient MOC2 (MOC2-STING+/-) or B78 (B78-STING-/-) tumors eliminated the effects of RT+AZD0156 on the expression of IFN-β, MHC-I, and PD-L1, and reduced CD8+T cell infiltration and migration. Additional anti-PD-L1 therapy promoted antitumor response by elevation of tumor-MHC-I and lymphocyte activation.
CONCLUSIONS: Combined radiation and AZD0156 increase STING-dependent antitumor response. Tumor-derived cell-autonomous IFN-β amplification drives both MHC-I and PD-L1 induction at the tumor cell surface, which is required by anti-PD-L1 therapy to promote antitumor immune response following RT and AZD0156 combination therapy.
PMID:37730275 | PMC:PMC10510866 | DOI:10.1136/jitc-2023-007474
View details for PubMedID 37730275
-
More
-
Radiation-associated secondary malignancies: a novel opportunity for applying immunotherapies Cancer immunology, immunotherapy : CII
Atajanova T, Rahman MM, Konieczkowski DJ, Morris ZS
2023 Nov;72(11):3445-3452. doi: 10.1007/s00262-023-03532-1. Epub 2023 Sep 2.
-
More
Radiation is commonly used as a treatment intended to cure or palliate cancer patients. Despite remarkable advances in the precision of radiotherapy delivery, even the most advanced forms inevitably expose some healthy tissues surrounding the target site to radiation. On rare occasions, this results in the development of radiation-associated secondary malignancies (RASM). RASM are typically high-grade and carry a poorer prognosis than their non-radiated counterparts. RASM are characterized by a high mutation burden, increased T cell infiltration, and a microenvironment that bears unique inflammatory signatures of prior radiation, including increased expression of various cytokines (e.g., TGF-β, TNF-α, IL4, and IL10). Interestingly, these cytokines have been shown to up-regulate the expression of PD-1 and/or PD-L1-an immune checkpoint receptor/ligand pair that is commonly targeted by immune checkpoint blocking immunotherapies. Here, we review the current understanding of the tumor-immune interactions in RASM, highlight the distinct clinical and molecular characteristics of RASM that may render them immunologically "hot," and propose a rationale for the formal testing of immune checkpoint blockade as a treatment approach for patients with RASM.
PMID:37658906 | PMC:PMC10992240 | DOI:10.1007/s00262-023-03532-1
View details for PubMedID 37658906
-
More
-
The Cancer Moonshot Immuno-Oncology Translational Network at 5: accelerating cancer immunotherapies Journal of the National Cancer Institute
Annapragada A, Sikora AG, Marathe H, Liu S, Demetriou M, Fong L, Gao J, Kufe D, Morris ZS, Vilar E, Sharon E, Hutson A, Odunsi K
2023 Nov 8;115(11):1262-1270. doi: 10.1093/jnci/djad151.
-
More
The Immuno-Oncology Translational Network (IOTN) was established in 2018 as part of the Cancer Moonshot. In 2022, President Joe Biden set new goals to reduce the cancer death rate by half within 25 years and improve the lives of people with cancer and cancer survivors. The IOTN is focused on accelerating translation of cancer immunology research, from bench to bedside, and improving immunotherapy outcomes across a wide array of cancers in the adult population. The unique structure and team science approach of the IOTN is designed to accelerate discovery and evaluation of novel immune-based therapeutic and prevention strategies. In this article, we describe IOTN progress to date, including new initiatives and the development of a robust set of resources to advance cancer immunology research. We summarize new insights by IOTN researchers, some of which are ripe for translation for several types of cancers. Looking to the future, we identify barriers to the translation of immuno-oncology concepts into clinical trials and key areas for action and improvements that are suitable for high-yield investments. Based on these experiences, we recommend novel National Institutes of Health funding mechanisms and development of new resources to address these barriers.
PMID:37572314 | PMC:PMC10637038 | DOI:10.1093/jnci/djad151
View details for PubMedID 37572314
-
More
-
Updates on radiotherapy-immunotherapy combinations: Proceedings of 6<sup>th</sup> annual ImmunoRad conference Oncoimmunology
Gregucci F, Spada S, Barcellos-Hoff MH, Bhardwaj N, Hak CW, Fiorentino A, Guha C, Guzman ML, Harrington K, Herrera FG, Honeychurch J, Hong T, Iturri L, Jaffee E, Karam SD, Knott RV, Koumenis C, Lyden D, Marciscano AE, Melcher A, Mondini M, Mondino A, Morris ZS, Pitroda S, Quezada SA, Santambrogio L, Shiao S, Stagg J, Telarovic I, Timmerman R, Vozenin M, Weichselbaum R, Welsh J, Wilkins A, Xu C, Zappasodi R, Zou W, Bobard A, Demaria S, Galluzzi L, Deutsch E, Formenti SC
2023 Jun 21;12(1):2222560. doi: 10.1080/2162402X.2023.2222560. eCollection 2023.
-
More
Focal radiation therapy (RT) has attracted considerable attention as a combinatorial partner for immunotherapy (IT), largely reflecting a well-defined, predictable safety profile and at least some potential for immunostimulation. However, only a few RT-IT combinations have been tested successfully in patients with cancer, highlighting the urgent need for an improved understanding of the interaction between RT and IT in both preclinical and clinical scenarios. Every year since 2016, ImmunoRad gathers experts working at the interface between RT and IT to provide a forum for education and discussion, with the ultimate goal of fostering progress in the field at both preclinical and clinical levels. Here, we summarize the key concepts and findings presented at the Sixth Annual ImmunoRad conference.
PMID:37363104 | PMC:PMC10286673 | DOI:10.1080/2162402X.2023.2222560
View details for PubMedID 37363104
-
More
-
Advancing Towards Personalized Prescription of Radiotherapy Dose Seminars in radiation oncology
Citrin D, Morris ZS
2023 Jul;33(3):219-220. doi: 10.1016/j.semradonc.2023.03.008.
-
Estrogen receptor blockade and radiation therapy cooperate to enhance the response of immunologically cold ER+ breast cancer to immunotherapy Breast cancer research : BCR
O'Leary KA, Bates AM, Jin WJ, Burkel BM, Sriramaneni RN, Emma SE, Nystuen EJ, Sumiec EG, Ponik SM, Morris ZS, Schuler LA
2023 Jun 13;25(1):68. doi: 10.1186/s13058-023-01671-y.
-
More
BACKGROUND: Most patients with estrogen receptor positive (ER+) breast cancer do not respond to immune checkpoint inhibition (ICI); the tumor microenvironment (TME) of these cancers is generally immunosuppressive and contains few tumor-infiltrating lymphocytes. Radiation therapy (RT) can increase tumor inflammation and infiltration by lymphocytes but does not improve responses to ICIs in these patients. This may result, in part, from additional effects of RT that suppress anti-tumor immunity, including increased tumor infiltration by myeloid-derived suppressor cells and regulatory T cells. We hypothesized that anti-estrogens, which are a standard of care for ER+ breast cancer, may ameliorate these detrimental effects of RT by reducing the recruitment/ activation of suppressive immune populations in the radiated TME, increasing anti-tumor immunity and responsiveness to ICIs.
METHODS: To interrogate the effect of the selective estrogen receptor downregulator, fulvestrant, on the irradiated TME in the absence of confounding growth inhibition by fulvestrant on tumor cells, we used the TC11 murine model of anti-estrogen resistant ER+ breast cancer. Tumors were orthotopically transplanted into immunocompetent syngeneic mice. Once tumors were established, we initiated treatment with fulvestrant or vehicle, followed by external beam RT one week later. We examined the number and activity of tumor infiltrating immune cells using flow cytometry, microscopy, transcript levels, and cytokine profiles. We tested whether fulvestrant improved tumor response and animal survival when added to the combination of RT and ICI.
RESULTS: Despite resistance of TC11 tumors to anti-estrogen therapy alone, fulvestrant slowed tumor regrowth following RT, and significantly altered multiple immune populations in the irradiated TME. Fulvestrant reduced the influx of Ly6C+Ly6G+ cells, increased markers of pro-inflammatory myeloid cells and activated T cells, and augmented the ratio of CD8+: FOXP3+ T cells. In contrast to the minimal effects of ICIs when co-treated with either fulvestrant or RT alone, combinatorial treatment with fulvestrant, RT and ICIs significantly reduced tumor growth and prolonged survival.
CONCLUSIONS: A combination of RT and fulvestrant can overcome the immunosuppressive TME in a preclinical model of ER+ breast cancer, enhancing the anti-tumor response and increasing the response to ICIs, even when growth of tumor cells is no longer estrogen sensitive.
PMID:37312163 | PMC:PMC10265911 | DOI:10.1186/s13058-023-01671-y
View details for PubMedID 37312163
-
More
-
Using <sup>18</sup>F-DCFPyL Prostate-Specific Membrane Antigen-Directed Positron Emission Tomography/Magnetic Resonance Imaging to Define Intraprostatic Boosts for Prostate Stereotactic Body Radiation Therapy Advances in radiation oncology
Floberg JM, Wells SA, Ojala D, Bayliss RA, Hill PM, Morris BA, Morris ZS, Ritter M, Cho SY
2023 Apr 9;8(5):101241. doi: 10.1016/j.adro.2023.101241. eCollection 2023 Sep-Oct.
-
More
PURPOSE: The recently reported FLAME trial demonstrated a biochemical disease-free survival benefit to using a focal intraprostatic boost to multiparametric magnetic resonance imaging (mpMRI)-identified lesions in men with localized prostate cancer treated with definitive radiation therapy. Prostate-specific membrane antigen (PSMA)-directed positron emission tomography (PET) may identify additional areas of disease. In this work, we investigated using both PSMA PET and mpMRI in planning focal intraprostatic boosts using stereotactic body radiation therapy (SBRT).
METHODS AND MATERIALS: We evaluated a cohort of patients (n = 13) with localized prostate cancer who were imaged with 2-(3-(1-carboxy-5-[(6-[18F]fluoro-pyridine-2-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL) PET/MRI on a prospective imaging trial before undergoing definitive therapy. The number of lesions concordant (overlapping) and discordant (no overlap) on PET and MRI was assessed. Overlap between concordant lesions was evaluated using the Dice and Jaccard similarity coefficients. Prostate SBRT plans were created fusing the PET/MRI imaging to computed tomography scans acquired the same day. Plans were created using only MRI-identified lesions, only PET-identified lesions, and the combined PET/MRI lesions. Coverage of the intraprostatic lesions and doses to the rectum and urethra were assessed for each of these plans.
RESULTS: The majority of lesions (21/39, 53.8%) were discordant between MRI and PET, with more lesions seen by PET alone (12) than MRI alone (9). Of lesions that were concordant between PET and MRI, there were still areas that did not overlap between scans (average Dice coefficient, 0.34). Prostate SBRT planning using all lesions to define a focal intraprostatic boost provided the best coverage of all lesions without compromising constraints on the rectum and urethra.
CONCLUSIONS: Using both mpMRI and PSMA-directed PET may better identify all areas of gross disease within the prostate. Using both imaging modalities could improve the planning of focal intraprostatic boosts.
PMID:37250282 | PMC:PMC10209128 | DOI:10.1016/j.adro.2023.101241
View details for PubMedID 37250282
-
More
-
<em>In Situ</em> Vaccination Following Intratumoral Injection of IL2 and Poly-l-lysine/Iron Oxide/CpG Nanoparticles to a Radiated Tumor Site ACS nano
Zhang Y, Rahman MM, Clark PA, Sriramaneni RN, Havighurst T, Kerr CP, Zhu M, Jones J, Wang X, Kim K, Gong S, Morris ZS
2023 Jun 13;17(11):10236-10251. doi: 10.1021/acsnano.3c00418. Epub 2023 May 22.
-
More
The in situ vaccine effect of radiation therapy (RT) has been shown to be limited in both preclinical and clinical settings, possibly due to the inadequacy of RT alone to stimulate in situ vaccination in immunologically "cold" tumor microenvironments (TMEs) and the mixed effects of RT in promoting tumor infiltration of both effector and suppressor immune cells. To address these limitations, we combined intratumoral injection of the radiated site with IL2 and a multifunctional nanoparticle (PIC). The local injection of these agents produced a cooperative effect that favorably immunomodulated the irradiated TME, enhancing the activation of tumor-infiltrating T cells and improving systemic anti-tumor T cell immunity. In syngeneic murine tumor models, the PIC+IL2+RT combination significantly improved the tumor response, surpassing the single or dual combinations of these treatments. Furthermore, this treatment led to the activation of tumor-specific immune memory and improved abscopal effects. Our findings suggest that this strategy can be used to augment the in situ vaccine effect of RT in clinical settings.
PMID:37216491 | PMC:PMC10278176 | DOI:10.1021/acsnano.3c00418
View details for PubMedID 37216491
-
More
-
Toxicity and Patient-Reported Quality-of-Life Outcomes After Prostate Stereotactic Body Radiation Therapy With Focal Boost to Magnetic Resonance Imaging-Identified Prostate Cancer Lesions: Results of a Phase 2 Trial International journal of radiation oncology, biology, physics
Morris BA, Holmes EE, Anger NJ, Cooley G, Schuster JM, Hurst N, Baschnagel AM, Bassetti MF, Blitzer GC, Chappell RJ, Bayliss RA, Morris ZS, Ritter MA, Floberg JM
2023 Nov 1;117(3):613-623. doi: 10.1016/j.ijrobp.2023.05.004. Epub 2023 May 12.
-
More
PURPOSE: In this prospective phase 2 trial, we investigated the toxicity and patient-reported quality-of-life outcomes in patients treated with stereotactic body radiation therapy (SBRT) to the prostate gland and a simultaneous focal boost to magnetic resonance imaging (MRI)-identified intraprostatic lesions while also de-escalating dose to the adjacent organs at risk.
METHODS AND MATERIALS: Eligible patients included low- or intermediate-risk prostate cancer (Gleason score ≤7, prostate specific antigen ≤20, T stage ≤2b). SBRT was prescribed to 40 Gy in 5 fractions delivered every other day to the prostate, with any areas of high disease burden (MRI-identified prostate imaging reporting and data system 4 or 5 lesions) simultaneously escalated to 42.5 to 45 Gy and areas overlapping organs at risk (within 2 mm of urethra, rectum, and bladder) constrained to 36.25 Gy (n = 100). Patients without a pretreatment MRI or without MRI-identified lesions were treated to dose of 37.5 Gy with no focal boost (n = 14).
RESULTS: From 2015 to 2022, a total of 114 patients were enrolled with a median follow-up of 42 months. No acute or late grade 3+ gastrointestinal (GI) toxicity was observed. One patient developed late grade 3 genitourinary (GU) toxicity at 16 months. In patients treated with focal boost (n = 100), acute grade 2 GU and GI toxicity was seen in 38% and 4% of patients, respectively. Cumulative late grade 2+ GU and GI toxicities at 24 months were 13% and 5% respectively. Patient-reported outcomes showed no significant long-term change from baseline in urinary, bowel, hormonal, or sexual quality-of-life scores after treatment.
CONCLUSIONS: SBRT to a dose of 40 Gy to the prostate gland with a simultaneous focal boost up to 45 Gy is well tolerated with similar rates of acute and late grade 2+ GI and GU toxicity as seen in other SBRT regimens without intraprostatic boost. Moreover, no significant long-term changes were seen in patient-reported urinary, bowel, or sexual outcomes from pretreatment baseline.
PMID:37179035 | DOI:10.1016/j.ijrobp.2023.05.004
View details for PubMedID 37179035
-
More
-
MRI-Guided Radiation Therapy Advances in oncology
Lee SL, Hall WA, Morris ZS, Christensen L, Bassetti M
2021 May;1:29-39. doi: 10.1016/j.yao.2021.02.003. Epub 2021 May 19.
-
Factors impacting the efficacy of the in-situ vaccine with CpG and OX40 agonist Cancer immunology, immunotherapy : CII
Pieper AA, Spiegelman DV, Felder AR, Feils AS, Tsarovsky NW, Zaborek J, Morris ZS, Erbe AK, Rakhmilevich AL, Sondel PM
2023 Jul;72(7):2459-2471. doi: 10.1007/s00262-023-03433-3. Epub 2023 Apr 5.
-
More
BACKGROUND: The in-situ vaccine using CpG oligodeoxynucleotide combined with OX40 agonist antibody (CpG + OX40) has been shown to be an effective therapy activating an anti-tumor T cell response in certain settings. The roles of tumor volume, tumor model, and the addition of checkpoint blockade in the efficacy of CpG + OX40 in-situ vaccination remains unknown.
METHODS: Mice bearing flank tumors (B78 melanoma or A20 lymphoma) were treated with combinations of CpG, OX40, and anti-CTLA-4. Tumor growth and survival were monitored. In vivo T cell depletion, tumor cell phenotype, and tumor infiltrating lymphocyte (TIL) studies were performed. Tumor cell sensitivity to CpG and macrophages were evaluated in vitro.
RESULTS: As tumor volumes increased in the B78 (one-tumor) and A20 (one-tumor or two-tumor) models, the anti-tumor efficacy of the in-situ vaccine decreased. In vitro, CpG had a direct effect on A20 proliferation and phenotype and an indirect effect on B78 proliferation via macrophage activation. As A20 tumors progressed in vivo, tumor cell phenotype changed, and T cells became more involved in the local CpG + OX40 mediated anti-tumor response. In mice with larger tumors that were poorly responsive to CpG + OX40, the addition of anti-CTLA-4 enhanced the anti-tumor efficacy in the A20 but not B78 models.
CONCLUSIONS: Increased tumor volume negatively impacts the anti-tumor capability of CpG + OX40 in-situ vaccine. The addition of checkpoint blockade augmented the efficacy of CpG + OX40 in the A20 but not B78 model. These results highlight the importance of considering multiple preclinical model conditions when assessing the efficacy of cancer immunotherapy regimens and their translation to clinical testing.
PMID:37016127 | PMC:PMC10264285 | DOI:10.1007/s00262-023-03433-3
View details for PubMedID 37016127
-
More
-
Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment Pharmaceutics
Kerr CP, Grudzinski JJ, Nguyen TP, Hernandez R, Weichert JP, Morris ZS
2022 Dec 30;15(1):128. doi: 10.3390/pharmaceutics15010128.
-
More
Targeted radionuclide therapy (TRT) and immunotherapy are rapidly growing classes of cancer treatments. Basic, translational, and clinical research are now investigating therapeutic combinations of these agents. In comparison to external beam radiation therapy (EBRT), TRT has the unique advantage of treating all disease sites following intravenous injection and selective tumor uptake and retention-a particularly beneficial property in metastatic disease settings. The therapeutic value of combining radiation therapy with immune checkpoint blockade to treat metastases has been demonstrated in preclinical studies, whereas results of clinical studies have been mixed. Several clinical trials combining TRT and immune checkpoint blockade have been initiated based on preclinical studies combining these with EBRT and/or TRT. Despite the interest in translation of TRT and immunotherapy combinations, many questions remain surrounding the mechanisms of interaction and the optimal approach to clinical implementation of these combinations. This review highlights the mechanisms of interaction between anti-tumor immunity and radiation therapy and the status of basic and translational research and clinical trials investigating combinations of TRT and immunotherapies.
PMID:36678756 | PMC:PMC9865370 | DOI:10.3390/pharmaceutics15010128
View details for PubMedID 36678756
-
More
-
Radiation to all macroscopic sites of tumor permits greater systemic antitumor response to in situ vaccination Journal for immunotherapy of cancer
Carlson PM, Patel RB, Birstler J, Rodriquez M, Sun C, Erbe AK, Bates AM, Marsh I, Grudzinski J, Hernandez R, Pieper AA, Feils AS, Rakhmilevich AL, Weichert JP, Bednarz BP, Sondel PM, Morris ZS
2023 Jan;11(1):e005463. doi: 10.1136/jitc-2022-005463.
-
More
BACKGROUND: The antitumor effects of external beam radiation therapy (EBRT) are mediated, in part, by an immune response. We have reported that a single fraction of 12 Gy EBRT combined with intratumoral anti-GD2 hu14.18-IL2 immunocytokine (IC) generates an effective in situ vaccine (ISV) against GD2-positive murine tumors. This ISV is effective in eradicating single tumors with sustained immune memory; however, it does not generate an adequate abscopal response against macroscopic distant tumors. Given the immune-stimulatory capacity of radiation therapy (RT), we hypothesized that delivering RT to all sites of disease would augment systemic antitumor responses to ISV.
METHODS: We used a syngeneic B78 murine melanoma model consisting of a 'primary' flank tumor and a contralateral smaller 'secondary' flank tumor, treated with 12 Gy EBRT and intratumoral IC immunotherapy to the primary and additional EBRT to the secondary tumor. As a means of delivering RT to all sites of disease, both known and occult, we also used a novel alkylphosphocholine analog, NM600, conjugated to 90Y as a targeted radionuclide therapy (TRT). Tumor growth, overall survival, and cause of death were measured. Flow cytometry was used to evaluate immune population changes in both tumors.
RESULTS: Abscopal effects of local ISV were amplified by delivering as little as 2-6 Gy of EBRT to the secondary tumor. When the primary tumor ISV regimen was delivered in mice receiving 12 Gy EBRT to the secondary tumor, we observed improved overall survival and more disease-free mice with immune memory compared with either ISV or 12 Gy EBRT alone. Similarly, TRT combined with ISV resulted in improved overall survival and a trend towards reduced tumor growth rates when compared with either treatment alone. Using flow cytometry, we identified an influx of CD8+ T cells with a less exhausted phenotype in both the ISV-targeted primary and the distant secondary tumor following the combination of secondary tumor EBRT or TRT with primary tumor ISV.
CONCLUSIONS: We report a novel use for low-dose RT, not as a direct antitumor modality but as an immunomodulator capable of driving and expanding antitumor immunity against metastatic tumor sites following ISV.
PMID:36639155 | PMC:PMC9843201 | DOI:10.1136/jitc-2022-005463
View details for PubMedID 36639155
-
More
-
Local TLR4 stimulation augments in situ vaccination induced via local radiation and anti-CTLA-4 checkpoint blockade through induction of CD8 T-cell independent Th1 polarization Journal for immunotherapy of cancer
Jagodinsky JC, Bates AM, Clark PA, Sriramaneni RN, Havighurst TC, Chakravarty I, Nystuen EJ, Kim K, Sondel PM, Jin WJ, Morris ZS
2022 Oct;10(10):e005103. doi: 10.1136/jitc-2022-005103.
-
More
BACKGROUND: Radiation therapy (RT) has been demonstrated to generate an in situ vaccination (ISV) effect in murine models and in patients with cancer; however, this has not routinely translated into enhanced clinical response to immune checkpoint inhibition (ICI). We investigated whether the commonly used vaccine adjuvant, monophosphoryl lipid A (MPL) could augment the ISV regimen consisting of combination RT and ICI.
MATERIALS/METHODS: We used syngeneic murine models of melanoma (B78) and prostate cancer (Myc-CaP). Tumor-bearing mice received either RT (12 Gy, day 1), RT+anti-CTLA-4 (C4, day 3, 6, 9), MPL (20 µg IT injection days 5, 7, 9), RT+C4+MPL, or PBS control. To evaluate the effect of MPL on the irradiated tumor microenvironment, primary tumor with tumor draining lymph nodes were harvested for immune cell infiltration analysis and cytokine profiling, and serum was collected for analysis of antitumor antibody populations.
RESULTS: Combination RT+C4+MPL significantly reduced tumor growth, increased survival and complete response rate compared with RT+C4 in both B78 and Myc-CaP models. MPL favorably reprogrammed the irradiated tumor-immune microenvironment toward M1 macrophage and Th1 TBET+CD4+ T cell polarization. Furthermore, MPL significantly increased intratumoral expression of several Th1-associated and M1-associated proinflammatory cytokines. In co-culture models, MPL-stimulated macrophages directly activated CD8 T cells and polarized CD4 cells toward Th1 phenotype. MPL treatment significantly increased production of Th1-associated, IgG2c antitumor antibodies, which were required for and predictive of antitumor response to RT+C4+MPL, and enabled macrophage-mediated antibody-dependent direct tumor cell killing by MPL-stimulated macrophages. Macrophage-mediated tumor cell killing was dependent on FcγR expression. In metastatic models, RT and MPL generated a systemic antitumor immune response that augmented response to ICIs. This was dependent on macrophages and CD4+ but not CD8+T cells.
CONCLUSIONS: We report the potential for MPL to augment the ISV effect of combination RT+C4 through FcγR, macrophage, and TBET+CD4+ Th1 cell dependent mechanisms. To our knowledge, this is the first report describing generation of a CD8+ T cell-independent, Th1 polarized, systemic antitumor immune response with subsequent generation of immunologic memory. These findings support the potential for vaccine adjuvants to enhance the efficacy of in situ tumor vaccine approaches.
PMID:36192087 | PMC:PMC9535200 | DOI:10.1136/jitc-2022-005103
View details for PubMedID 36192087
-
More
-
Multifunctional nanoparticle potentiates the in situ vaccination effect of radiation therapy and enhances response to immune checkpoint blockade Nature communications
Zhang Y, Sriramaneni RN, Clark PA, Jagodinsky JC, Ye M, Jin W, Wang Y, Bates A, Kerr CP, Le T, Allawi R, Wang X, Xie R, Havighurst TC, Chakravarty I, Rakhmilevich AL, O'Leary KA, Schuler LA, Sondel PM, Kim K, Gong S, Morris ZS
2022 Aug 23;13(1):4948. doi: 10.1038/s41467-022-32645-x.
-
More
Radiation therapy (RT) activates an in situ vaccine effect when combined with immune checkpoint blockade (ICB), yet this effect may be limited because RT does not fully optimize tumor antigen presentation or fully overcome suppressive mechanisms in the tumor-immune microenvironment. To overcome this, we develop a multifunctional nanoparticle composed of polylysine, iron oxide, and CpG (PIC) to increase tumor antigen presentation, increase the ratio of M1:M2 tumor-associated macrophages, and enhance stimulation of a type I interferon response in conjunction with RT. In syngeneic immunologically "cold" murine tumor models, the combination of RT, PIC, and ICB significantly improves tumor response and overall survival resulting in cure of many mice and consistent activation of tumor-specific immune memory. Combining RT with PIC to elicit a robust in situ vaccine effect presents a simple and readily translatable strategy to potentiate adaptive anti-tumor immunity and augment response to ICB or potentially other immunotherapies.
PMID:35999216 | PMC:PMC9399096 | DOI:10.1038/s41467-022-32645-x
View details for PubMedID 35999216
-
More
-
Mechanism of effective combination radio-immunotherapy against 9464D-GD2, an immunologically cold murine neuroblastoma Journal for immunotherapy of cancer
Aiken TJ, Erbe AK, Zebertavage L, Komjathy D, Feils AS, Rodriguez M, Stuckwisch A, Gillies SD, Morris ZS, Birstler J, Rakhmilevich AL, Sondel PM
2022 May;10(5):e004834. doi: 10.1136/jitc-2022-004834.
-
More
BACKGROUND: Most pediatric cancers are considered immunologically cold with relatively few responding to immune checkpoint inhibition. We recently described an effective combination radio-immunotherapy treatment regimen ( c ombination a daptive- i nnate immunotherapy r egimen (CAIR)) targeting adaptive and innate immunity in 9464D-GD2, an immunologically cold model of neuroblastoma. Here, we characterize the mechanism of CAIR and the role of major histocompatibility complex class I (MHC-I) in the treatment response.
METHODS: Mice bearing GD2-expressing 9464D-GD2 tumors were treated with CAIR (external beam radiotherapy, hu14.18-IL2 immunocytokine, CpG, anti-CD40, and anti-CTLA4) and tumor growth and survival were tracked. Depletion of specific immune cell lineages, as well as testing in immunodeficient R2G2 mice, were used to determine the populations necessary for treatment efficacy. Induction of MHC-I expression in 9464D-GD2 cells in response to interferon-γ (IFN-γ) and CAIR was measured in vitro and in vivo, respectively, by flow cytometry and quantitative real-time PCR. A cell line with IFN-γ-inducible MHC-I expression (9464D-GD2-I) was generated by transfecting a subclone of the parental cell line capable of expressing MHC-I with GD2 synthase and was used in vivo to assess the impact of MHC-I expression on responsiveness to CAIR.
RESULTS: CAIR cures some mice bearing small (50 mm3) but not larger (100 mm3) 9464D-GD2 tumors and these cured mice develop weak memory responses against tumor rechallenge. Early suppression of 9464D-GD2 tumors by CAIR does not require T or natural killer (NK) cells, but eventual tumor cures are NK cell dependent. Unlike the parental 9464D cell line, 9464D-GD2 cells have uniformly very low MHC-I expression at baseline and fail to upregulate expression in response to IFN-γ. In contrast, 9464D-GD2-I upregulates MHC-I in response to IFN-γ and is less responsive to CAIR.
CONCLUSION: Treatment with CAIR cures 9464D-GD2 tumors in a NK cell dependent manner and induction of MHC-I by tumors cells was associated with decreased efficacy. These results demonstrate that the early tumor response to this regimen is T and NK cell independent, but that NK cells have a role in generating lasting cures in the absence of MHC-I expression by tumor cells. Further strategies to better inhibit tumor outgrowth in this setting may require further NK activation or the ability to engage alternative immune effector cells.
PMID:35618290 | PMC:PMC9125770 | DOI:10.1136/jitc-2022-004834
View details for PubMedID 35618290
-
More
-
The Role of MRI-Guided Radiotherapy for Soft Tissue Sarcomas Journal of clinical medicine
Blitzer GC, Yadav P, Morris ZS
2022 Feb 17;11(4):1042. doi: 10.3390/jcm11041042.
-
More
Soft tissue sarcomas (STS) are a rare class of tumors that originate from mesenchymal tissues and occur most frequently in the extremities, trunk, and retroperitoneum. Surgical resection with R0 margins is the primary curative treatment for most localized STS. In this setting, radiation therapy is used either pre-operatively or post-operatively to reduce the rate of local recurrence. Modern pre- or post-operative radiation therapy rely on the use of MRI sequences to guide target delineation during treatment planning. MRI-guided radiotherapy also offers unique advantages over CT-guided approaches in differentiating STS from surrounding normal soft tissues and enabling better identification of target volumes on daily imaging. For patients with unresectable STS, radiation therapy may offer the best chance for local tumor control. However, most STS are relatively radioresistant with modest rates of local control achieved using conventionally fractionated radiation. Specialized techniques such as hypofractionated radiation may allow for dose intensification and may increase rates of local control for STS. In these settings, MRI becomes even more critical for the delineation of targets and organs at risk and management of tumor and organ at risk motion during and between radiotherapy treatment fractions.
PMID:35207317 | PMC:PMC8880805 | DOI:10.3390/jcm11041042
View details for PubMedID 35207317
-
More
-
Radiation Augments the Local Anti-Tumor Effect of <em>In Situ</em> Vaccine With CpG-Oligodeoxynucleotides and Anti-OX40 in Immunologically Cold Tumor Models Frontiers in immunology
Pieper AA, Zangl LM, Speigelman DV, Feils AS, Hoefges A, Jagodinsky JC, Felder MA, Tsarovsky NW, Arthur IS, Brown RJ, Birstler J, Le T, Carlson PM, Bates AM, Hank JA, Rakhmilevich AL, Erbe AK, Sondel PM, Patel RB, Morris ZS
2021 Nov 15;12:763888. doi: 10.3389/fimmu.2021.763888. eCollection 2021.
-
More
INTRODUCTION: Combining CpG oligodeoxynucleotides with anti-OX40 agonist antibody (CpG+OX40) is able to generate an effective in situ vaccine in some tumor models, including the A20 lymphoma model. Immunologically "cold" tumors, which are typically less responsive to immunotherapy, are characterized by few tumor infiltrating lymphocytes (TILs), low mutation burden, and limited neoantigen expression. Radiation therapy (RT) can change the tumor microenvironment (TME) of an immunologically "cold" tumor. This study investigated the effect of combining RT with the in situ vaccine CpG+OX40 in immunologically "cold" tumor models.
METHODS: Mice bearing flank tumors (A20 lymphoma, B78 melanoma or 4T1 breast cancer) were treated with combinations of local RT, CpG, and/or OX40, and response to treatment was monitored. Flow cytometry and quantitative polymerase chain reaction (qPCR) experiments were conducted to study differences in the TME, secondary lymphoid organs, and immune activation after treatment.
RESULTS: An in situ vaccine regimen of CpG+OX40, which was effective in the A20 model, did not significantly improve tumor response or survival in the "cold" B78 and 4T1 models, as tested here. In both models, treatment with RT prior to CpG+OX40 enabled a local response to this in situ vaccine, significantly improving the anti-tumor response and survival compared to RT alone or CpG+OX40 alone. RT increased OX40 expression on tumor infiltrating CD4+ non-regulatory T cells. RT+CpG+OX40 increased the ratio of tumor-infiltrating effector T cells to T regulatory cells and significantly increased CD4+ and CD8+ T cell activation in the tumor draining lymph node (TDLN) and spleen.
CONCLUSION: RT significantly improves the local anti-tumor effect of the in situ vaccine CpG+OX40 in immunologically "cold", solid, murine tumor models where RT or CpG+OX40 alone fail to stimulate tumor regression.
PMID:34868010 | PMC:PMC8634717 | DOI:10.3389/fimmu.2021.763888
View details for PubMedID 34868010
-
More
-
Toward magnetic resonance fingerprinting for low-field MR-guided radiation therapy Medical physics
Mickevicius NJ, Kim JP, Zhao J, Morris ZS, Hurst NJ, Glide-Hurst CK
2021 Nov;48(11):6930-6940. doi: 10.1002/mp.15202. Epub 2021 Sep 18.
-
More
PURPOSE: The acquisition of multiparametric quantitative magnetic resonance imaging (qMRI) is becoming increasingly important for functional characterization of cancer prior to- and throughout the course of radiation therapy. The feasibility of a qMRI method known as magnetic resonance fingerprinting (MRF) for rapid T1 and T2 mapping was assessed on a low-field MR-linac system.
METHODS: A three-dimensional MRF sequence was implemented on a 0.35T MR-guided radiotherapy system. MRF-derived measurements of T1 and T2 were compared to those obtained with gold standard single spin echo methods, and the impacts of the radiofrequency field homogeneity and scan times ranging between 6 and 48 min were analyzed by acquiring between 1 and 8 spokes per time point in a standard quantitative system phantom. The short-term repeatability of MRF was assessed over three measurements taken over a 10-h period. To evaluate transferability, MRF measurements were acquired on two additional MR-guided radiotherapy systems. Preliminary human volunteer studies were performed.
RESULTS: The phantom benchmarking studies showed that MRF is capable of mapping T1 and T2 values within 8% and 10% of gold standard measures, respectively, at 0.35T. The coefficient of variation of T1 and T2 estimates over three repeated scans was < 5% over a broad range of relaxation times. The T1 and T2 times derived using a single-spoke MRF acquisition across three scanners were near unity and mean percent errors in T1 and T2 estimates using the same phantom were < 3%. The mean percent differences in T1 and T2 as a result of truncating the scan time to 6 min over the large range of relaxation times in the system phantom were 0.65% and 4.05%, respectively.
CONCLUSIONS: The technical feasibility and accuracy of MRF on a low-field MR-guided radiation therapy device has been demonstrated. MRF can be used to measure accurate T1 and T2 maps in three dimensions from a brief 6-min scan, offering strong potential for efficient and reproducible qMRI for future clinical trials in functional plan adaptation and tumor/normal tissue response assessment.
PMID:34487357 | PMC:PMC8733901 | DOI:10.1002/mp.15202
View details for PubMedID 34487357
-
More
-
Prospective Clinical Investigation of the Efficacy of Combination Radiation Therapy With Immune Checkpoint Inhibition International journal of radiation oncology, biology, physics
Akama-Garren EH, Morris ZS, Sikora AG, Weichselbaum R, Schoenfeld JD
2021 Dec 1;111(5):1165-1175. doi: 10.1016/j.ijrobp.2021.08.009. Epub 2021 Aug 16.
-
More
Immune checkpoint inhibitors (ICIs) lead to durable responses in a subset of patients with cancer, but most patients do not respond to ICI, prompting interest in combining immunotherapy with other therapeutic regimens. Preclinical evidence supports the potential for therapeutic synergy between immunotherapy and radiation therapy through modulation of the tumor microenvironment and antitumor immune responses. Local therapy also has the potential to overcome localized sites of relative immune suppression and resistance. Prospective clinical trials have been initiated to test these hypotheses in the clinic as well as to investigate the toxicities and adverse events associated with combination immunotherapy and radiation therapy. In this review, we discuss the emerging results from prospective clinical trials of combination immunotherapy and radiation therapy, the safety and efficacy of their combination, concordance with preclinical and retrospective data, and some of the remaining open questions to be addressed by future clinical trials.
PMID:34411638 | PMC:PMC10960630 | DOI:10.1016/j.ijrobp.2021.08.009
View details for PubMedID 34411638
-
More
-
Safety and feasibility of an in situ vaccination and immunomodulatory targeted radionuclide combination immuno-radiotherapy approach in a comparative (companion dog) setting PloS one
Magee K, Marsh IR, Turek MM, Grudzinski J, Aluicio-Sarduy E, Engle JW, Kurzman ID, Zuleger CL, Oseid EA, Jaskowiak C, Albertini MR, Esbona K, Bednarz B, Sondel PM, Weichert JP, Morris ZS, Hernandez R, Vail DM
2021 Aug 12;16(8):e0255798. doi: 10.1371/journal.pone.0255798. eCollection 2021.
-
More
RATIONALE: Murine syngeneic tumor models have revealed efficacious systemic antitumor responses following primary tumor in situ vaccination combined with targeted radionuclide therapy to secondary or metastatic tumors. Here we present studies on the safety and feasibility of this approach in a relevant translational companion dog model (n = 17 dogs) with advanced cancer.
METHODS: The three component of the combination immuno-radiotherapy approach were employed either separately or in combination in companion dogs with advanced stage cancer. In situ vaccination was achieved through the administration of hypofractionated external beam radiotherapy and intratumoral hu14.18-IL2 fusion immunocytokine injections to the index tumor. In situ vaccination was subsequently combined with targeted radionuclide therapy using a theranostic pairing of IV 86Y-NM600 (for PET imaging and subject-specific dosimetry) and IV 90Y-NM600 (therapeutic radionuclide) prescribed to deliver an immunomodulatory 2 Gy dose to all metastatic sites in companion dogs with metastatic melanoma or osteosarcoma. In a subset of dogs, immunologic parameters preliminarily assessed.
RESULTS: The components of the immuno-radiotherapy combination were well tolerated either alone or in combination, resulting in only transient low grade (1 or 2) adverse events with no dose-limiting events observed. In subject-specific dosimetry analyses, we observed 86Y-NM600 tumor:bone marrow absorbed-dose differential uptakes ≥2 in 4 of 5 dogs receiving the combination, which allowed subsequent safe delivery of at least 2 Gy 90Y-NM600 TRT to tumors. NanoString gene expression profiling and immunohistochemistry from pre- and post-treatment biopsy specimens provide evidence of tumor microenvironment immunomodulation by 90Y-NM600 TRT.
CONCLUSIONS: The combination of external beam radiotherapy, intratumoral immunocytokine, and targeted radionuclide immuno-radiotherapy known to have activity against syngeneic melanoma in murine models is feasible and well tolerated in companion dogs with advanced stage, spontaneously arising melanoma or osteosarcoma and has immunomodulatory potential. Further studies evaluating the dose-dependent immunomodulatory effects of this immuno-radiotherapy combination are currently ongoing.
PMID:34383787 | PMC:PMC8360580 | DOI:10.1371/journal.pone.0255798
View details for PubMedID 34383787
-
More
-
Targeted Alpha-Particle Radiotherapy and Immune Checkpoint Inhibitors Induces Cooperative Inhibition on Tumor Growth of Malignant Melanoma Cancers
Li M, Liu D, Lee D, Cheng Y, Baumhover NJ, Marks BM, Sagastume EA, Ballas ZK, Johnson FL, Morris ZS, Schultz MK
2021 Jul 22;13(15):3676. doi: 10.3390/cancers13153676.
-
More
Radiotherapy can facilitate the immune recognition of immunologically "cold" tumors and enhance the efficacy of anti-PD-1 and anti-CTLA-4 immune checkpoint inhibitors (ICIs) in melanoma. Systemic administration of receptor-targeted radionuclide therapy has the potential to selectively deliver radionuclides to multiple tumors throughout the body in metastatic settings. By triggering immunologic cell death and increasing the immune susceptibility of surviving tumor cells in these locations, targeted radionuclide therapies may overcome resistance to ICIs and render immunologically "cold" tumors throughout the body responsive to ICIs and immunologically "hot". Here, we show the anti-tumor cooperation of targeted α-particle radionuclide therapy (α-TRT) and ICIs in preclinical models of melanoma. Melanocortin 1 receptor (MC1R)-targeted radiopeptide [212Pb]VMT01 was employed to deliver α-radiation to melanoma tumors in mice. A single injection of 4.1 MBq [212Pb]VMT01 significantly slowed the tumor growth of B16-F10 melanoma and the combination of [212Pb]VMT01 and ICIs induced a cooperative anti-tumor effect leading to 43% complete tumor response with no sign of malignancy on autopsy. Animals with complete response developed anti-tumor immunity to reject further tumor inoculations. This therapeutic cooperation was completely abolished in RAG1 KO mice, which are deficient in T-cell maturation. In addition, the anti-tumor cooperation was compromised when fractionated [212Pb]VMT01 was used in the combination. We also demonstrated that [212Pb]VMT01 induced immunogenic cell death in tumor vaccination assays and in vitro exposure to [212Pb]VMT01 sensitized immunotolerant melanoma to ICIs treatment in vivo. Enhanced tumor infiltrating CD3+, CD4+, CD8+ lymphocytes were observed following injection of 1.4 MBq [212Pb]VMT01. Overall, we demonstrated anti-tumor cooperation between α-TRT and ICIs in melanoma that is mediated by tumor specific immunity.
PMID:34359580 | PMC:PMC8345035 | DOI:10.3390/cancers13153676
View details for PubMedID 34359580
-
More
-
Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade Science translational medicine
Patel RB, Hernandez R, Carlson P, Grudzinski J, Bates AM, Jagodinsky JC, Erbe A, Marsh IR, Arthur I, Aluicio-Sarduy E, Sriramaneni RN, Jin WJ, Massey C, Rakhmilevich AL, Vail D, Engle JW, Le T, Kim K, Bednarz B, Sondel PM, Weichert J, Morris ZS
2021 Jul 14;13(602):eabb3631. doi: 10.1126/scitranslmed.abb3631.
-
More
Molecular and cellular effects of radiotherapy on tumor microenvironment (TME) can help prime and propagate antitumor immunity. We hypothesized that delivering radiation to all tumor sites could augment response to immunotherapies. We tested an approach to enhance response to immune checkpoint inhibitors (ICIs) by using targeted radionuclide therapy (TRT) to deliver radiation semiselectively to tumors. NM600, an alkylphosphocholine analog that preferentially accumulates in most tumor types, chelates a radioisotope and semiselectively delivers it to the TME for therapeutic or diagnostic applications. Using serial 86Y-NM600 positron emission tomography (PET) imaging, we estimated the dosimetry of 90Y-NM600 in immunologically cold syngeneic murine models that do not respond to ICIs alone. We observed strong therapeutic efficacy and reported optimal dose (2.5 to 5 gray) and sequence for 90Y-NM600 in combination with ICIs. After combined treatment, 45 to 66% of mice exhibited complete response and tumor-specific T cell memory, compared to 0% with 90Y-NM600 or ICI alone. This required expression of STING in tumor cells. Combined TRT and ICI activated production of proinflammatory cytokines in the TME, promoted tumor infiltration by and clonal expansion of CD8+ T cells, and reduced metastases. In mice bearing multiple tumors, combining TRT with moderate-dose (12 gray) external beam radiotherapy (EBRT) targeting a single tumor augmented response to ICIs compared to combination of ICIs with either TRT or EBRT alone. The safety of TRT was confirmed in a companion canine study. Low-dose TRT represents a translatable approach to promote response to ICIs for many tumor types, regardless of location.
PMID:34261797 | PMC:PMC8449934 | DOI:10.1126/scitranslmed.abb3631
View details for PubMedID 34261797
-
More
-
Optimizing Flow Cytometric Analysis of Immune Cells in Samples Requiring Cryopreservation from Tumor-Bearing Mice Journal of immunology (Baltimore, Md. : 1950)
Carlson PM, Mohan M, Patel RB, Birstler J, Nettenstrom L, Sheerar D, Fox K, Rodriguez M, Hoefges A, Hernandez R, Zahm C, Kim K, McNeel DG, Weichert J, Morris ZS, Sondel PM
2021 Jul 15;207(2):720-734. doi: 10.4049/jimmunol.2000656. Epub 2021 Jul 14.
-
More
Most shared resource flow cytometry facilities do not permit analysis of radioactive samples. We are investigating low-dose molecular targeted radionuclide therapy (MTRT) as an immunomodulator in combination with in situ tumor vaccines and need to analyze radioactive samples from MTRT-treated mice using flow cytometry. Further, the sudden shutdown of core facilities in response to the COVID-19 pandemic has created an unprecedented work stoppage. In these and other research settings, a robust and reliable means of cryopreservation of immune samples is required. We evaluated different fixation and cryopreservation protocols of disaggregated tumor cells with the aim of identifying a protocol for subsequent flow cytometry of the thawed sample, which most accurately reflects the flow cytometric analysis of the tumor immune microenvironment of a freshly disaggregated and analyzed sample. Cohorts of C57BL/6 mice bearing B78 melanoma tumors were evaluated using dual lymphoid and myeloid immunophenotyping panels involving fixation and cryopreservation at three distinct points during the workflow. Results demonstrate that freezing samples after all staining and fixation are completed most accurately matches the results from noncryopreserved equivalent samples. We observed that cryopreservation of living, unfixed cells introduces a nonuniform alteration to PD1 expression. We confirm the utility of our cryopreservation protocol by comparing tumors treated with in situ tumor vaccines, analyzing both fresh and cryopreserved tumor samples with similar results. Last, we use this cryopreservation protocol with radioactive specimens to demonstrate potentially beneficial effector cell changes to the tumor immune microenvironment following administration of a novel MTRT in a dose- and time-dependent manner.
PMID:34261667 | PMC:PMC8313010 | DOI:10.4049/jimmunol.2000656
View details for PubMedID 34261667
-
More
-
Quantification and molecular imaging of fatty acid isomers from complex biological samples by mass spectrometry Chemical science
Zhang H, Xu M, Shi X, Liu Y, Li Z, Jagodinsky JC, Ma M, Welham NV, Morris ZS, Li L
2021 May 4;12(23):8115-8122. doi: 10.1039/d1sc01614h.
-
More
Elucidating the isomeric structure of free fatty acids (FAs) in biological samples is essential to comprehend their biological functions in various physiological and pathological processes. Herein, we report a novel approach of using peracetic acid (PAA) induced epoxidation coupled with mass spectrometry (MS) for localization of the C[double bond, length as m-dash]C bond in unsaturated FAs, which enables both quantification and spatial visualization of FA isomers from biological samples. Abundant diagnostic fragment ions indicative of the C[double bond, length as m-dash]C positions were produced upon fragmentation of the FA epoxides derived from either in-solution or on-tissue PAA epoxidation of free FAs. The performance of the proposed approach was evaluated by analysis of FAs in human cell lines as well as mapping the FA isomers from cancer tissue samples with MALDI-TOF/TOF-MS. Merits of the newly developed method include high sensitivity, simplicity, high reaction efficiency, and capability of spatial characterization of FA isomers in tissue samples.
PMID:34194701 | PMC:PMC8208125 | DOI:10.1039/d1sc01614h
View details for PubMedID 34194701
-
More
-
Combination of radiation therapy, bempegaldesleukin, and checkpoint blockade eradicates advanced solid tumors and metastases in mice Journal for immunotherapy of cancer
Pieper AA, Rakhmilevich AL, Spiegelman DV, Patel RB, Birstler J, Jin WJ, Carlson PM, Charych DH, Hank JA, Erbe AK, Overwijk WW, Morris ZS, Sondel PM
2021 Jun;9(6):e002715. doi: 10.1136/jitc-2021-002715.
-
More
BACKGROUND: Current clinical trials are using radiation therapy (RT) to enhance an antitumor response elicited by high-dose interleukin (IL)-2 therapy or immune checkpoint blockade (ICB). Bempegaldesleukin (BEMPEG) is an investigational CD122-preferential IL-2 pathway agonist with prolonged in vivo half-life and preferential intratumoral expansion of T effector cells over T regulatory cells. BEMPEG has shown encouraging safety and efficacy in clinical trials when used in combination with PD-1 checkpoint blockade. In this study, we investigated the antitumor effect of local RT combined with BEMPEG in multiple immunologically 'cold' tumor models. Additionally, we asked if ICB could further enhance the local and distant antitumor effect of RT+BEMPEG in the setting of advanced solid tumors or metastatic disease.
METHODS: Mice bearing flank tumors (B78 melanoma, 4T1 breast cancer, or MOC2 head and neck squamous cell carcinoma) were treated with combinations of RT and immunotherapy (including BEMPEG, high-dose IL-2, anti(α)-CTLA-4, and α-PD-L1). Mice bearing B78 flank tumors were injected intravenously with B16 melanoma cells to mimic metastatic disease and were subsequently treated with RT and/or immunotherapy. Tumor growth and survival were monitored. Peripheral T cells and tumor-infiltrating lymphocytes were assessed via flow cytometry.
RESULTS: A cooperative antitumor effect was observed in all models when RT was combined with BEMPEG, and RT increased IL-2 receptor expression on peripheral T cells. This cooperative interaction was associated with increased IL-2 receptor expression on peripheral T cells following RT. In the B78 melanoma model, RT+BEMPEG resulted in complete tumor regression in the majority of mice with a single ~400 mm3 tumor. This antitumor response was T-cell dependent and supported by long-lasting immune memory. Adding ICB to RT+BEMPEG strengthened the antitumor response and cured the majority of mice with a single ~1000 mm3 B78 tumor. In models with disseminated metastasis (B78 primary with B16 metastasis, 4T1, and MOC2), the triple combination of RT, BEMPEG, and ICB significantly improved primary tumor response and survival.
CONCLUSION: The combination of local RT, BEMPEG, and ICB cured mice with advanced, immunologically cold tumors and distant metastasis in a T cell-dependent manner, suggesting this triple combination warrants clinical testing.
PMID:34172518 | PMC:PMC8237721 | DOI:10.1136/jitc-2021-002715
View details for PubMedID 34172518
-
More
-
Future Directions in the Use of SAbR for the Treatment of Oligometastatic Cancers Seminars in radiation oncology
Morris Z, Dohopolski M, Rahimi A, Timmerman R
2021 Jul;31(3):253-262. doi: 10.1016/j.semradonc.2021.03.004.
-
More
The role of local therapy as a sole therapy or part of a combined approach in treating metastatic cancer continues to evolve. The most obvious requirements for prudent implementation of local therapies like stereotactic ablative radiotherapy (SAbR) to become mainstream in treating oligometastases are (1) Clear guidance as to what particular patients might benefit, and (2) Confirmation of improvements in outcome after such treatments via clinical trials. These future directional requirements are non-negotiable. However, innovation and research offer many more opportunities to understand and improve therapy. Identifying candidates and personalizing their therapy can be afforded via proteomic, genomic and epigenomic characterization techniques. Such molecular profiling along with liquid biopsy opportunities will both help select best therapies and facilitate ongoing monitoring of response. Technologies both to find targets and help deliver less-toxic therapy continue to improve and will be available in the marketplace. These technologies include molecular-based imaging (eg, PET-PSMA), FLASH ultra-high dose rate platforms, Grid therapy, PULSAR adaptive dosing, and MRI/PET guided linear accelerators. Importantly, a treatment approach beyond oligometastastic could evolve including a rationale for using SAbR in the oligoprogressive, oligononresponsive, oligobulky and oligolethal settings as well as expansion beyond oligo- toward even plurimetastastic disease. In any case, lessons learned and experiences required by the implementation of using SAbR in oligometastatic cancer will be revisited.
PMID:34090653 | DOI:10.1016/j.semradonc.2021.03.004
View details for PubMedID 34090653
-
More
-
Using Radiation Therapy to Prime and Propagate an Anti-tumor Immune Response Against Brain Tumors Neuromolecular medicine
Onate AJ, Clark PA, Morris ZS
2022 Mar;24(1):3-7. doi: 10.1007/s12017-021-08668-w. Epub 2021 Jun 3.
-
More
Immunotherapies have demonstrated efficacy and survival benefits in some patients suffering from brain tumors; however, most do not respond and new approaches to enhance anti-tumor immunotherapeutic responses in the brain are needed. Radiotherapy remains a commonly used cancer treatment modality and can augment immunotherapeutic responses through multiple mechanisms. Recent preclinical studies may provide insight on how to optimally combine radiation and immunotherapies to maximize treatment efficacy. Unique aspects of the brain tumor microenvironment may play a critical role in limiting the successful application of immunotherapies in this location. Emerging studies suggest that such limits may be redressed through combination of immunotherapies with radiation therapy. In these settings, the latter may play a critical role in immunomodulating both tumor cells and the radiated brain tumor microenvironment. This review analyzes recent developments in combining radiation and immunotherapies to prime and better propagate anti-tumor immune response against brain tumors.
PMID:34081276 | PMC:PMC8639822 | DOI:10.1007/s12017-021-08668-w
View details for PubMedID 34081276
-
More
-
Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy Theranostics
Jagodinsky JC, Jin WJ, Bates AM, Hernandez R, Grudzinski JJ, Marsh IR, Chakravarty I, Arthur IS, Zangl LM, Brown RJ, Nystuen EJ, Emma SE, Kerr C, Carlson PM, Sriramaneni RN, Engle JW, Aluicio-Sarduy E, Barnhart TE, Le T, Kim K, Bednarz BP, Weichert JP, Patel RB, Morris ZS
2021 Apr 15;11(13):6120-6137. doi: 10.7150/thno.54881. eCollection 2021.
-
More
Rationale: Clinical interest in combining targeted radionuclide therapies (TRT) with immunotherapies is growing. External beam radiation therapy (EBRT) activates a type 1 interferon (IFN1) response mediated via stimulator of interferon genes (STING), and this is critical to its therapeutic interaction with immune checkpoint blockade. However, little is known about the time course of IFN1 activation after EBRT or whether this may be induced by decay of a TRT source. Methods: We examined the IFN1 response and expression of immune susceptibility markers in B78 and B16 melanomas and MOC2 head and neck cancer murine models using qPCR and western blot. For TRT, we used 90Y chelated to NM600, an alkylphosphocholine analog that exhibits selective uptake and retention in tumor cells including B78 and MOC2. Results: We observed significant IFN1 activation in all cell lines, with peak activation in B78, B16, and MOC2 cell lines occurring 7, 7, and 1 days, respectively, following RT for all doses. This effect was STING-dependent. Select IFN response genes remained upregulated at 14 days following RT. IFN1 activation following STING agonist treatment in vitro was identical to RT suggesting time course differences between cell lines were mediated by STING pathway kinetics and not DNA damage susceptibility. In vivo delivery of EBRT and TRT to B78 and MOC2 tumors resulted in a comparable time course and magnitude of IFN1 activation. In the MOC2 model, the combination of 90Y-NM600 and dual checkpoint blockade therapy reduced tumor growth and prolonged survival compared to single agent therapy and cumulative dose equivalent combination EBRT and dual checkpoint blockade therapy. Conclusions: We report the time course of the STING-dependent IFN1 response following radiation in multiple murine tumor models. We show the potential of TRT to stimulate IFN1 activation that is comparable to that observed with EBRT and this may be critical to the therapeutic integration of TRT with immunotherapies.
PMID:33995649 | PMC:PMC8120207 | DOI:10.7150/thno.54881
View details for PubMedID 33995649
-
More
-
Combination of Bempegaldesleukin and Anti-CTLA-4 Prevents Metastatic Dissemination After Primary Resection or Radiotherapy in a Preclinical Model of Non-Small Cell Lung Cancer Frontiers in oncology
Bates AM, Brown RJ, Pieper AA, Zangl LM, Arthur I, Carlson PM, Le T, Sosa GA, Clark PA, Sriramaneni RN, Kim K, Patel RB, Morris ZS
2021 Apr 15;11:645352. doi: 10.3389/fonc.2021.645352. eCollection 2021.
-
More
Surgical resection or hypo-fractionated radiation therapy (RT) in early-stage non-small cell lung cancer (NSCLC) achieves local tumor control, but metastatic relapse remains a challenge. We hypothesized that immunotherapy with anti-CTLA-4 and bempegaldesleukin (BEMPEG; NKTR-214), a CD122-preferential IL2 pathway agonist, after primary tumor RT or resection would reduce metastases in a syngeneic murine NSCLC model. Mice bearing Lewis Lung Carcinoma (LLC) tumors were treated with combinations of BEMPEG, anti-CTLA-4, and primary tumor treatment (surgical resection or RT). Primary tumor size, mouse survival, and metastatic disease at the time of death were assessed. Flow cytometry, qRT-PCR, and cytokine analyses were performed on tumor specimens. All mice treated with RT or surgical resection of primary tumor alone succumbed to metastatic disease, and all mice treated with BEMPEG and/or anti-CTLA-4 succumbed to primary tumor local progression. The combination of primary tumor RT or resection and BEMPEG and anti-CTLA-4 reduced spontaneous metastasis and improved survival without any noted toxicity. Flow cytometric immunoprofiling of primary tumors revealed increased CD8 T and NK cells and decreased T-regulatory cells with the combination of BEMPEG, anti-CTLA-4, and RT compared to RT alone. Increased expression of genes associated with tumor cell immune susceptibility, immune cell recruitment, and cytotoxic T lymphocyte activation were observed in tumors of mice treated with BEMPEG, anti-CTLA-4, and RT. The combination of BEMPEG and anti-CTLA-4 with primary tumor RT or resection enabled effective control of local and metastatic disease in a preclinical murine NSCLC model. This therapeutic combination has important translational potential for patients with early-stage NSCLC and other cancers.
PMID:33937052 | PMC:PMC8083981 | DOI:10.3389/fonc.2021.645352
View details for PubMedID 33937052
-
More
-
Depth of tumor implantation affects response to in situ vaccination in a syngeneic murine melanoma model Journal for immunotherapy of cancer
Carlson PM, Mohan M, Rodriguez M, Subbotin V, Sun CX, Patel RB, Birstler J, Hank JA, Rakhmilevich AL, Morris ZS, Erbe AK, Sondel PM
2021 Apr;9(4):e002107. doi: 10.1136/jitc-2020-002107.
-
More
An important component of research using animal models is ensuring rigor and reproducibility. This study was prompted after two experimenters performing virtually identical studies obtained different results when syngeneic B78 murine melanoma cells were implanted into the skin overlying the flank and treated with an in situ vaccine (ISV) immunotherapy. Although both experimenters thought they were using identical technique, we determined that one was implanting the tumors intradermally (ID) and the other was implanting them subcutaneously (SC). Though the baseline in vivo immunogenicity of tumors can depend on depth of their implantation, the response to immunotherapy as a function of tumor depth, particularly in immunologically 'cold' tumors, has not been well studied. The goal of this study was to evaluate the difference in growth kinetics and response to immunotherapy between identically sized melanoma tumors following ID versus SC implantation. We injected C57BL/6 mice with syngeneic B78 melanoma cells either ID or SC in the flank. When tumors reached 190-230 mm3, they were grouped into a 'wave' and treated with our previously published ISV regimen (12 Gy local external beam radiation and intratumoral hu14.18-IL2 immunocytokine). Physical examination demonstrated that ID-implanted tumors were mobile on palpation, while SC-implanted tumors became fixed to the underlying fascia. Histologic examination identified a critical fascial layer, the panniculus carnosus, which separated ID and SC tumors. SC tumors reached the target tumor volume significantly faster compared with ID tumors. Most ID tumors exhibited either partial or complete response to this immunotherapy, whereas most SC tumors did not. Further, the 'mobile' or 'fixed' phenotype of tumors predicted response to therapy, regardless of intended implantation depth. These findings were then extended to additional immunotherapy regimens in four separate tumor models. These data indicate that the physical 'fixed' versus 'mobile' characterization of the tumors may be one simple method of ensuring homogeneity among implanted tumors prior to initiation of treatment. Overall, this short report demonstrates that small differences in depth of tumor implantation can translate to differences in response to immunotherapy, and proposes a simple physical examination technique to ensure consistent tumor depth when conducting implantable tumor immunotherapy experiments.
PMID:33858849 | PMC:PMC8055108 | DOI:10.1136/jitc-2020-002107
View details for PubMedID 33858849
-
More
-
Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? Journal for immunotherapy of cancer
Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, Crittenden M, Shiao S, Khleif S, Gupta S, Formenti SC, Vikram B, Coleman CN, Ahmed MM
2021 Apr;9(4):e002038. doi: 10.1136/jitc-2020-002038.
-
More
Recent evidence indicates that ionizing radiation can enhance immune responses to tumors. Advances in radiation delivery techniques allow hypofractionated delivery of conformal radiotherapy. Hypofractionation or other modifications of standard fractionation may improve radiation's ability to promote immune responses to tumors. Other novel delivery options may also affect immune responses, including T-cell activation and tumor-antigen presentation changes. However, there is limited understanding of the immunological impact of hypofractionated and unique multifractionated radiotherapy regimens, as these observations are relatively recent. Hence, these differences in radiotherapy fractionation result in distinct immune-modulatory effects. Radiation oncologists and immunologists convened a virtual consensus discussion to identify current deficiencies, challenges, pitfalls and critical gaps when combining radiotherapy with immunotherapy and making recommendations to the field and advise National Cancer Institute on new directions and initiatives that will help further development of these two fields.This commentary aims to raise the awareness of this complexity so that the need to study radiation dose, fractionation, type and volume is understood and valued by the immuno-oncology research community. Divergence of approaches and findings between preclinical studies and clinical trials highlights the need for evaluating the design of future clinical studies with particular emphasis on radiation dose and fractionation, immune biomarkers and selecting appropriate end points for combination radiation/immune modulator trials, recognizing that direct effect on the tumor and potential abscopal effect may well be different. Similarly, preclinical studies should be designed as much as possible to model the intended clinical setting. This article describes a conceptual framework for testing different radiation therapy regimens as separate models of how radiation itself functions as an immunomodulatory 'drug' to provide alternatives to the widely adopted 'one-size-fits-all' strategy of frequently used 8 Gy×3 regimens immunomodulation.
PMID:33827904 | PMC:PMC8031689 | DOI:10.1136/jitc-2020-002038
View details for PubMedID 33827904
-
More
-
Low-Dose Radiation Potentiates the Propagation of Anti-Tumor Immunity against Melanoma Tumor in the Brain after In Situ Vaccination at a Tumor outside the Brain Radiation research
Clark PA, Sriramaneni RN, Bates AM, Jin WJ, Jagodinsky JC, Hernandez R, Le T, Jeffery JJ, Marsh IR, Grudzinski JJ, Aluicio-Sarduy E, Barnhart TE, Anderson BR, Chakravarty I, Arthur IS, Kim K, Engle JW, Bednarz BP, Weichert JP, Morris ZS
2021 Jun 1;195(6):522-540. doi: 10.1667/RADE-20-00237.1.
-
More
Brain metastases develop in over 60% of advanced melanoma patients and negatively impact quality of life and prognosis. In a murine melanoma model, we previously showed that an in situ vaccination (ISV) regimen, combining radiation treatment and intratumoral (IT) injection of immunocytokine (IC: anti-GD2 antibody fused to IL2), along with the immune checkpoint inhibitor anti-CTLA-4, robustly eliminates peripheral flank tumors but only has modest effects on co-occurring intracranial tumors. In this study, we investigated the ability of low-dose radiation to the brain to potentiate anti-tumor immunity against a brain tumor when combined with ISV + anti-CTLA-4. B78 (GD2+, immunologically "cold") melanoma tumor cells were implanted into the flank and the right striatum of the brain in C57BL/6 mice. Flank tumors (50-150 mm3) were treated following a previously optimized ISV regimen [radiation (12 Gy × 1, treatment day 1), IT-IC (50 µg daily, treatment days 6-10), and anti-CTLA-4 (100 µg, treatment days 3, 6, 9)]. Mice that additionally received whole-brain radiation treatment (WBRT, 4 Gy × 1) on day 15 demonstrated significantly increased survival compared to animals that received ISV + anti-CTLA-4 alone, WBRT alone or no treatment (control) (P < 0.001, log-rank test). Timing of WBRT was critical, as WBRT administration on day 1 did not significantly enhance survival compared to ISV + anti-CTLA-4, suggesting that the effect of WBRT on survival might be mediated through immune modulation and not just direct tumor cell cytotoxicity. Modest increases in T cells (CD8+ and CD4+) and monocytes/macrophages (F4/80+) but no changes in FOXP3+ regulatory T cells (Tregs), were observed in brain melanoma tumors with addition of WBRT (on day 15) to ISV + anti-CTLA-4. Cytokine multiplex immunoassay revealed distinct changes in both intracranial melanoma and contralateral normal brain with addition of WBRT (day 15) to ISV + anti-CTLA-4, with notable significant changes in pro-inflammatory (e.g., IFNγ, TNFα and LIX/CXCL5) and suppressive (e.g., IL10, IL13) cytokines as well as chemokines (e.g., IP-10/CXCL10 and MIG/CXCL9). We tested the ability of the alkylphosphocholine analog, NM600, to deliver immunomodulatory radiation to melanoma brain tumors as a targeted radionuclide therapy (TRT). Yttrium-86 (86Y) chelated to NM600 was delivered intravenously by tail vein to mice harboring flank and brain melanoma tumors, and PET imaging demonstrated specific accumulation up to 72 h at each tumor site (∼12:1 brain tumor/brain and ∼8:1 flank tumor/muscle). When NM600 was chelated to therapeutic β-particle-emitting 90Y and administered on treatment day 13, T-cell infiltration and cytokine profiles were altered in melanoma brain tumor, like that observed for WBRT. Overall, our results demonstrate that addition of low-dose radiation, timed appropriately with ISV administration to tumors outside the brain, significantly increases survival in animals co-harboring melanoma brain tumors. This observation has potentially important translational implications as a treatment strategy for increasing the response of tumors in the brain to systemically administered immunotherapies.
PMID:33826741 | PMC:PMC8259713 | DOI:10.1667/RADE-20-00237.1
View details for PubMedID 33826741
-
More
-
A multipurpose brachytherapy catheter to enable intratumoral injection Brachytherapy
Jagodinsky JC, Medeiros G, Raj HH, Razuan A, Locsin A, Dempsey TG, Tang B, Chakravarty I, Clark PA, Sriramaneni RN, Jin WJ, Lan K, Das RK, Miller JR, Suarez-Gonzalez D, Morris ZS
2021 Jul-Aug;20(4):900-910. doi: 10.1016/j.brachy.2020.10.012. Epub 2021 Mar 27.
-
More
PURPOSE: To create and test a multipurpose brachytherapy catheter prototype enabling intratumoral injection and brachytherapy after a single catheter insertion.
METHODS AND MATERIALS: The design of the prototype consists of an outer tube and an inner syringe tube that can be filled with injectable agent. The outer sheath and inner syringe tube were constructed using polytetrafluoroethylene tubing, and the other components were 3D printed using dental resin and polylactic acid material. To demonstrate functionality, we injected in vitro phantoms with dyed saline. For proof of concept, we demonstrated the potential for the prototype to deliver cell therapy, enhance tumor delineation, deliver tattoo ink for pathology marking, avoid toxicity through local delivery of chemotherapy, and facilitate combination brachytherapy and immunotherapy.
RESULTS: The prototype enables accurate injection in vitro and in vivo without altering dosimetry. To illustrate the potential for delivery of cell therapies, we injected luciferase-expressing splenocytes and confirmed their delivery with bioluminescence imaging. To demonstrate feasibility of radiographically visualizing injected material, we delivered iohexol contrast intratumorally and confirmed tumor retention using Faxitron x-ray imaging. In addition, we show the potential of intratumoral administration to reduce toxicity associated with cyclophosphamide compared with systemic administration. To demonstrate feasibility, we treated tumor-bearing mice with brachytherapy (192Ir source, 2 Gy to 5 mm) in combination with intratumoral injection of 375,000 U of interleukin 2 and observed no increased toxicity.
CONCLUSIONS: These results demonstrate that a prototype multipurpose brachytherapy catheter enables accurate intratumoral injection and support the feasibility of combining intratumoral injection with brachytherapy.
PMID:33785280 | PMC:PMC8323107 | DOI:10.1016/j.brachy.2020.10.012
View details for PubMedID 33785280
-
More
-
Tumor-Specific Antibody, Cetuximab, Enhances the <em>In Situ</em> Vaccine Effect of Radiation in Immunologically Cold Head and Neck Squamous Cell Carcinoma Frontiers in immunology
Jin WJ, Erbe AK, Schwarz CN, Jaquish AA, Anderson BR, Sriramaneni RN, Jagodinsky JC, Bates AM, Clark PA, Le T, Lan K, Chen Y, Kim K, Morris ZS
2020 Nov 12;11:591139. doi: 10.3389/fimmu.2020.591139. eCollection 2020.
-
More
In head and neck squamous cell carcinoma (HNSCC) tumors that over-expresses huEGFR, the anti-EGFR antibody, cetuximab, antagonizes tumor cell viability and sensitizes to radiation therapy. However, the immunologic interactions between cetuximab and radiation therapy are not well understood. We transduced two syngeneic murine HNSCC tumor cell lines to express human EGFR (MOC1- and MOC2-huEGFR) in order to facilitate evaluation of the immunologic interactions between radiation and cetuximab. Cetuximab was capable of inducing antibody-dependent cellular cytotoxicity (ADCC) in MOC1- and MOC2-huEGFR cells but showed no effect on the viability or radiosensitivity of these tumor cells, which also express muEGFR that is not targeted by cetuximab. Radiation enhanced the susceptibility of MOC1- and MOC2-huEGFR to ADCC, eliciting a type I interferon response and increasing expression of NKG2D ligands on these tumor cells. Co-culture of splenocytes with cetuximab and MOC2-huEGFR cells resulted in increased expression of IFNγ in not only NK cells but also in CD8+ T cells, and this was dependent upon splenocyte expression of FcγR. In MOC2-huEGFR tumors, combining radiation and cetuximab induced tumor growth delay that required NK cells, EGFR expression, and FcγR on host immune cells. Combination of radiation and cetuximab increased tumor infiltration with NK and CD8+ T cells but not regulatory T cells. Expression of PD-L1 was increased in MOC2-huEGFR tumors following treatment with radiation and cetuximab. Delivering anti-PD-L1 antibody with radiation and cetuximab improved survival and resulted in durable tumor regression in some mice. Notably, these cured mice showed evidence of an adaptive memory response that was not specifically directed against huEGFR. These findings suggest an opportunity to improve the treatment of HNSCC by combining radiation and cetuximab to engage an innate anti-tumor immune response that may prime an effective adaptive immune response when combined with immune checkpoint blockade. It is possible that this approach could be extended to any immunologically cold tumor that does not respond to immune checkpoint blockade alone and for which a tumor-specific antibody exists or could be developed.
PMID:33281820 | PMC:PMC7689006 | DOI:10.3389/fimmu.2020.591139
View details for PubMedID 33281820
-
More
-
The Radiobiology of Radiopharmaceuticals Seminars in radiation oncology
Morris ZS, Wang AZ, Knox SJ
2021 Jan;31(1):20-27. doi: 10.1016/j.semradonc.2020.07.002.
-
More
Radiopharmaceutical therapy or targeted radionuclide therapy (TRT) is a well-established class of cancer therapeutics that includes a growing number of FDA-approved drugs and a promising pipeline of experimental therapeutics. Radiobiology is fundamental to a mechanistic understanding of the therapeutic capacity of these agents and their potential toxicities. However, the field of radiobiology has historically focused on external beam radiation. Critical differences exist between TRT and external beam radiotherapy with respect to dosimetry, dose rate, linear energy transfer, duration of treatment delivery, fractionation, range, and target volume. These distinctions simultaneously make it difficult to extrapolate from the radiobiology of external beam radiation to that of TRT and pose considerable challenges for preclinical and clinical studies investigating TRT. Here, we discuss these challenges and explore the current understanding of the radiobiology of radiopharmaceuticals.
PMID:33246632 | PMC:PMC7990047 | DOI:10.1016/j.semradonc.2020.07.002
View details for PubMedID 33246632
-
More
-
Intratumoral injection reduces toxicity and antibody-mediated neutralization of immunocytokine in a mouse melanoma model Journal for immunotherapy of cancer
Baniel CC, Sumiec EG, Hank JA, Bates AM, Erbe AK, Pieper AA, Hoefges AG, Patel RB, Rakhmilevich AL, Morris ZS, Sondel PM
2020 Oct;8(2):e001262. doi: 10.1136/jitc-2020-001262.
-
More
BACKGROUND: Some patients with cancer treated with anticancer monoclonal antibodies (mAbs) develop antidrug antibodies (ADAs) that recognize and bind the therapeutic antibody. This response may neutralize the therapeutic mAb, interfere with mAb effector function or cause toxicities. We investigated the potential influence of ADA to modify the tumor-binding capability of a tumor-reactive 'immunocytokine' (IC), namely, a fusion protein (hu14.18-IL2) consisting of a humanized, tumor-reactive, anti-GD2 mAb genetically linked to interleukin 2. We characterize the role of treatment delivery of IC (intravenous vs intratumoral) on the impact of ADA on therapeutic outcome following IC treatments in an established antimelanoma (MEL) regimen involving radiotherapy (RT) +IC.
METHODS: C57BL/6 mice were injected with human IgG or the hu14.18-IL2 IC to develop a mouse anti-human antibody (MAHA) response (MAHA+). In vitro assays were performed to assess ADA binding to IC using sera from MAHA+ and MAHA- mice. In vivo experiments assessed the levels of IC bound to tumor in MAHA+ and MAHA- mice, and the influence of IC route of delivery on its ability to bind to B78 (GD2+) MEL tumors.
RESULTS: MAHA is inducible in C57BL/6 mice. In vitro assays show that MAHA is capable of inhibiting the binding of IC to GD2 antigen on B78 cells, resulting in impaired ADCC mediated by IC. When B78-bearing mice are injected intravenously with IC, less IC binds to B78-MEL tumors in MAHA+ mice than in MAHA- mice. In contrast, when IC is injected intratumorally in tumor-bearing mice, the presence of MAHA does not detectibly impact IC binding to the tumor. Combination therapy with RT+IT-IC showed improved tumor regression compared with RT alone in MAHA+ mice. If given intratumorally, IC could be safely readministered in tumor-bearing MAHA+ mice, while intravenous injections of IC in MAHA+ mice caused severe toxicity. Histamine levels were elevated in MAHA+ mice compared with MAHA- mice after reintroduction of IC.
CONCLUSIONS: Intratumoral injection may be a means of overcoming ADA neutralization of therapeutic activity of tumor-reactive mAbs or ICs and may reduce systemic toxicity, which could have significant translational relevance.
PMID:33115944 | PMC:PMC7594540 | DOI:10.1136/jitc-2020-001262
View details for PubMedID 33115944
-
More
-
<em>In situ</em> Vaccine Plus Checkpoint Blockade Induces Memory Humoral Response Frontiers in immunology
Baniel CC, Heinze CM, Hoefges A, Sumiec EG, Hank JA, Carlson PM, Jin WJ, Patel RB, Sriramaneni RN, Gillies SD, Erbe AK, Schwarz CN, Pieper AA, Rakhmilevich AL, Sondel PM, Morris ZS
2020 Jul 24;11:1610. doi: 10.3389/fimmu.2020.01610. eCollection 2020.
-
More
In a syngeneic murine melanoma (MEL) model, we recently reported an in situ vaccination response to combined radiation (RT) and intra-tumoral (IT) injection of anti-GD2 hu14. 18-IL2 immunocytokine (IC). This combined treatment resulted in 71% complete and durable regression of 5-week tumors, a tumor-specific memory T cell response, and augmented response to systemic anti-CTLA-4 antibody checkpoint blockade. While the ability of radiation to diversify anti-tumor T cell response has been reported, we hypothesize that mice rendered disease-free (DF) by a RT-based ISV might also exhibit a heightened B cell response. C57BL/6 mice were engrafted with 2 × 106 GD2+ B78 MEL and treated at a target tumor size of ~200 mm3 with 12 Gy RT, IT-IC on day (D)6-D10, and anti-CTLA-4 on D3, 6, and 9. Serum was collected via facial vein before tumor injection, before treatment, during treatment, after becoming DF, and following rejection of subcutaneous 2 × 106 B78 MEL re-challenge on D90. Flow cytometry demonstrated the presence of tumor-specific IgG in sera from mice rendered DF and rejecting re-challenge with B78 MEL at D90 after starting treatment. Consistent with an adaptive endogenous anti-tumor humoral memory response, these anti-tumor antibodies bound to B78 cells and parental B16 cells (GD2-), but not to the unrelated syngeneic Panc02 or Panc02 GD2+ cell lines. We evaluated the kinetics of this response and observed that tumor-specific IgG was consistently detected by D22 after initiation of treatment, corresponding to a time of rapid tumor regression. The amount of tumor-specific antibody binding to tumor cells (as measured by flow MFI) did not correlate with host animal prognosis. Incubation of B16 MEL cells in DF serum, vs. naïve serum, prior to IV injection, did not delay engraftment of B16 metastases and showed similar overall survival rates. B cell depletion using anti-CD20 or anti-CD19 and anti-B220 did not impact the efficacy of ISV treatment. Thus, treatment with RT + IC + anti-CTLA-4 results in adaptive anti-tumor humoral memory response. This endogenous tumor-specific antibody response does not appear to have therapeutic efficacy but may serve as a biomarker for an anti-tumor T cell response.
PMID:32849544 | PMC:PMC7396490 | DOI:10.3389/fimmu.2020.01610
View details for PubMedID 32849544
-
More
-
In situ vaccination at a peripheral tumor site augments response against melanoma brain metastases Journal for immunotherapy of cancer
Clark PA, Sriramaneni RN, Jin WJ, Jagodinsky JC, Bates AM, Jaquish AA, Anderson BR, Le T, Lubin JA, Chakravarty I, Arthur IS, Heinze CM, Guy EI, Kler J, Klar KA, Carlson PM, Kim KM, Kuo JS, Morris ZS
2020 Jul;8(2):e000809. doi: 10.1136/jitc-2020-000809.
-
More
BACKGROUND: Immune checkpoint inhibition (ICI) alone is not efficacious for a large number of patients with melanoma brain metastases. We previously established an in situ vaccination (ISV) regimen combining radiation and immunocytokine to enhance response to ICIs. Here, we tested whether ISV inhibits the development of brain metastases in a murine melanoma model.
METHODS: B78 (GD2+) melanoma 'primary' tumors were engrafted on the right flank of C57BL/6 mice. After 3-4 weeks, primary tumors were treated with ISV (radiation (12 Gy, day 1), α-GD2 immunocytokine (hu14.18-IL2, days 6-10)) and ICI (α-CTLA-4, days 3, 6, 9). Complete response (CR) was defined as no residual tumor observed at treatment day 90. Mice with CR were tested for immune memory by re-engraftment with B78 in the left flank and then the brain. To test ISV efficacy against metastases, tumors were also engrafted in the left flank and brain of previously untreated mice. Tumors were analyzed by quantitative reverse transcription-PCR, immunohistochemistry, flow cytometry and multiplex cytokine assay.
RESULTS: ISV+α-CTLA-4 resulted in immune memory and rejection of B78 engraftment in the brain in 11 of 12 mice. When B78 was engrafted in brain prior to treatment, ISV+α-CTLA-4 increased survival compared with ICI alone. ISV+α-CTLA-4 eradicated left flank tumors but did not elicit CR at brain sites when tumor cells were engrafted in brain prior to ISV. ISV+α-CTLA-4 increased CD8+ and CD4+ T cells in flank and brain tumors compared with untreated mice. Among ISV + α-CTLA-4 treated mice, left flank tumors showed increased CD8+ infiltration and CD8+:FOXP3+ ratio compared with brain tumors. Flank and brain tumors showed minimal differences in expression of immune checkpoint receptors/ligands or Mhc-1. Cytokine productions were similar in left flank and brain tumors in untreated mice. Following ISV+α-CTLA-4, production of immune-stimulatory cytokines was greater in left flank compared with brain tumor grafts.
CONCLUSION: ISV augmented response to ICIs in murine melanoma at brain and extracranial tumor sites. Although baseline tumor-immune microenvironments were similar at brain and extracranial tumor sites, response to ISV+α-CTLA-4 was divergent with reduced infiltration and activation of immune cells in brain tumors. Additional therapies may be needed for effective antitumor immune response against melanoma brain metastases.
PMID:32690669 | PMC:PMC7371368 | DOI:10.1136/jitc-2020-000809
View details for PubMedID 32690669
-
More
-
Cancer Moonshot Immuno-Oncology Translational Network (IOTN): accelerating the clinical translation of basic discoveries for improving immunotherapy and immunoprevention of cancer Journal for immunotherapy of cancer
Annapragada A, Sikora A, Bollard C, Conejo-Garcia J, Cruz CR, Demehri S, Demetriou M, Demirdjian L, Fong L, Horowitz M, Hutson A, Kadash-Edmondson K, Kufe D, Lipkin S, Liu S, McCarthy C, Morgan M, Morris Z, Pan Y, Pasquini M, Schoenberger S, Van Allen E, Vilar E, Xing Y, Zha W, Consortium I, Odunsi A
2020 Jun;8(1):e000796. doi: 10.1136/jitc-2020-000796.
-
More
Despite regulatory approval of several immune-based treatments for cancer in the past decade, a number of barriers remain to be addressed in order to fully harness the therapeutic potential of the immune system and provide benefits for patients with cancer. As part of the Cancer Moonshot initiative, the Immuno-Oncology Translational Network (IOTN) was established to accelerate the translation of basic discoveries to improve immunotherapy outcomes across the spectrum of adult cancers and to develop immune-based approaches that prevent cancers before they occur. The IOTN currently consists of 32 academic institutions in the USA. By leveraging cutting-edge preclinical research in immunotherapy and immunoprevention, open data and resource sharing, and fostering highly collaborative team science across the immuno-oncology ecosystem, the IOTN is designed to accelerate the generation of novel mechanism-driven immune-based cancer prevention and therapies, and the development of safe and effective personalized immuno-oncology approaches.
PMID:32554617 | PMC:PMC7304845 | DOI:10.1136/jitc-2020-000796
View details for PubMedID 32554617
-
More
-
Priming and Propagating Anti-tumor Immunity: Focal Hypofractionated Radiation for in Situ Vaccination and Systemic Targeted Radionuclide Theranostics for Immunomodulation of Tumor Microenvironments Seminars in radiation oncology
Jagodinsky JC, Morris ZS
2020 Apr;30(2):181-186. doi: 10.1016/j.semradonc.2019.12.008.
-
More
Recent preclinical and clinical studies have elucidated mechanisms whereby radiation therapy influences the anti-tumor immune response. Immunogenic cell death and phenotypic changes in tumor cells surviving radiation may underlie this effect and contribute to the capacity of radiation to elicit an in situ tumor vaccine effect. In situ vaccination is a therapeutic strategy that seeks to convert a patient's own tumor into a source of enhanced antigen recognition for the purpose of augmenting a systemic anti-tumor immune response. Capitalizing on the in situ vaccine effect of radiation, several groups have demonstrated anti-tumor efficacy in preclinical models by combining radiation with immune checkpoint blockade. Local delivery of immune adjuvants and/or immune stimulatory cytokines via direct injection into the radiated tumor microenvironment may further increase the in situ vaccine capacity of radiation therapy. However, recent studies suggest that in some contexts this effect is antagonized by the presence of distant untreated sites of disease that may dampen the systemic immune response generated by in situ vaccination through a phenomenon termed concomitant immune tolerance. Concomitant immune tolerance may be overcome by delivering radiation to all sites of metastatic disease, however this is often not possible to safely achieve using external beam radiation therapy without considerable risk of lymphopenia that would negate the immune effects of in situ vaccination. For patients with widespread metastatic disease, alternative strategies may include systemic treatment with targeted radionuclide therapies alone or in combination with an external beam radiation therapy-based in situ vaccine approach.
PMID:32381297 | PMC:PMC7286051 | DOI:10.1016/j.semradonc.2019.12.008
View details for PubMedID 32381297
-
More
-
The Promise of Combining Radiation Therapy With Immunotherapy International journal of radiation oncology, biology, physics
Jagodinsky JC, Harari PM, Morris ZS
2020 Sep 1;108(1):6-16. doi: 10.1016/j.ijrobp.2020.04.023. Epub 2020 Apr 23.
-
More
The development of immunotherapy in oncology builds upon many years of scientific investigation into the cellular mechanics underlying interactions between tumor cells and immune cell populations. The past decade has brought an accelerating pace to the clinical investigation of new immunotherapy agents, particularly in the setting of metastatic disease. The integration of immunotherapy into phase 3 clinical trial design has lagged in settings of advanced locoregional disease, where combination with radiation therapy may be critical. Yet, such may be the settings where immunotherapies have their greatest potential to affect patient survival and achieve curative outcomes. In this review, we discuss the interaction of radiation with the immune system and the potential to augment antitumor immunity through combined-modality approaches that integrate radiation and immunotherapies. The dynamics of cellular and tumor response to radiation offer unique opportunities for beneficial interplay with immunotherapy that may go unrecognized with conventional screening and monotherapy clinical testing of novel pharmaceutical agents. Using immune checkpoint blockade as a primary example, we discuss recent preclinical and clinical studies that illustrate the potential synergy of such therapies in combination with radiation, and we highlight the potential clinical value of such interactions. For various immunotherapy agents, their greatest clinical effect may rest in combination with radiation, and efforts to facilitate systematic investigation of this approach are highly warranted.
PMID:32335187 | PMC:PMC7442714 | DOI:10.1016/j.ijrobp.2020.04.023
View details for PubMedID 32335187
-
More
-
Outcome-Related Signatures Identified by Whole Transcriptome Sequencing of Resectable Stage III/IV Melanoma Evaluated after Starting Hu14.18-IL2 Clinical cancer research : an official journal of the American Association for Cancer Research
Yang RK, Kuznetsov IB, Ranheim EA, Wei JS, Sindiri S, Gryder BE, Gangalapudi V, Song YK, Patel V, Hank JA, Zuleger C, Erbe AK, Morris ZS, Quale R, Kim K, Albertini MR, Khan J, Sondel PM
2020 Jul 1;26(13):3296-3306. doi: 10.1158/1078-0432.CCR-19-3294. Epub 2020 Mar 9.
-
More
PURPOSE: We analyzed whole transcriptome sequencing in tumors from 23 patients with stage III or IV melanoma from a pilot trial of the anti-GD2 immunocytokine, hu14.18-IL2, to identify predictive immune and/or tumor biomarkers in patients with melanoma at high risk for recurrence.
EXPERIMENTAL DESIGN: Patients were randomly assigned to receive the first of three monthly courses of hu14.18-IL2 immunotherapy either before (Group A) or after (Group B) complete surgical resection of all known diseases. Tumors were evaluated by histology and whole transcriptome sequencing.
RESULTS: Tumor-infiltrating lymphocyte (TIL) levels directly associated with relapse-free survival (RFS) and overall survival (OS) in resected tumors from Group A, where early responses to the immunotherapy agent could be assessed. TIL levels directly associated with a previously reported immune signature, which associated with RFS and OS, particularly in Group A tumors. In Group A tumors, there were decreased cell-cycling gene RNA transcripts, but increased RNA transcripts for repair and growth genes. We found that outcome (RFS and OS) was directly associated with several immune signatures and immune-related RNA transcripts and inversely associated with several tumor growth-associated transcripts, particularly in Group A tumors. Most of these associations were not seen in Group B tumors.
CONCLUSIONS: We interpret these data to signify that both immunologic and tumoral cell processes, as measured by RNA-sequencing analyses detected shortly after initiation of hu14.18-IL2 therapy, are associated with long-term survival and could potentially be used as prognostic biomarkers in tumor resection specimens obtained after initiating neoadjuvant immunotherapy.
PMID:32152202 | PMC:PMC7334053 | DOI:10.1158/1078-0432.CCR-19-3294
View details for PubMedID 32152202
-
More
-
Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition Journal for immunotherapy of cancer
Voeller J, Erbe AK, Slowinski J, Rasmussen K, Carlson PM, Hoefges A, VandenHeuvel S, Stuckwisch A, Wang X, Gillies SD, Patel RB, Farrel A, Rokita JL, Maris J, Hank JA, Morris ZS, Rakhmilevich AL, Sondel PM
2019 Dec 6;7(1):344. doi: 10.1186/s40425-019-0823-6.
-
More
BACKGROUND: Unlike some adult cancers, most pediatric cancers are considered immunologically cold and generally less responsive to immunotherapy. While immunotherapy has already been incorporated into standard of care treatment for pediatric patients with high-risk neuroblastoma, overall survival remains poor. In a mouse melanoma model, we found that radiation and tumor-specific immunocytokine generate an in situ vaccination response in syngeneic mice bearing large tumors. Here, we tested whether a novel immunotherapeutic approach utilizing radiation and immunocytokine together with innate immune stimulation could generate a potent antitumor response with immunologic memory against syngeneic murine neuroblastoma.
METHODS: Mice bearing disialoganglioside (GD2)-expressing neuroblastoma tumors (either NXS2 or 9464D-GD2) were treated with radiation and immunotherapy (including anti-GD2 immunocytokine with or without anti-CTLA-4, CpG and anti-CD40 monoclonal antibody). Tumor growth, animal survival and immune cell infiltrate were analyzed in the tumor microenvironment in response to various treatment regimens.
RESULTS: NXS2 had a moderate tumor mutation burden (TMB) while N-MYC driven 9464D-GD2 had a low TMB, therefore the latter served as a better model for high-risk neuroblastoma (an immunologically cold tumor). Radiation and immunocytokine induced a potent in situ vaccination response against NXS2 tumors, but not in the 9464D-GD2 tumor model. Addition of checkpoint blockade with anti-CTLA-4 was not effective alone against 9464D-GD2 tumors; inclusion of CpG and anti-CD40 achieved a potent antitumor response with decreased T regulatory cells within the tumors and induction of immunologic memory.
CONCLUSIONS: These data suggest that a combined innate and adaptive immunotherapeutic approach can be effective against immunologically cold syngeneic murine neuroblastoma. Further testing is needed to determine how these concepts might translate into development of more effective immunotherapeutic approaches for the treatment of clinically high-risk neuroblastoma.
PMID:31810498 | PMC:PMC6898936 | DOI:10.1186/s40425-019-0823-6
View details for PubMedID 31810498
-
More
-
Development of an In Situ Cancer Vaccine via Combinational Radiation and Bacterial-Membrane-Coated Nanoparticles Advanced materials (Deerfield Beach, Fla.)
Patel RB, Ye M, Carlson PM, Jaquish A, Zangl L, Ma B, Wang Y, Arthur I, Xie R, Brown RJ, Wang X, Sriramaneni R, Kim K, Gong S, Morris ZS
2019 Oct;31(43):e1902626. doi: 10.1002/adma.201902626. Epub 2019 Sep 16.
-
More
Neoantigens induced by random mutations and specific to an individual's cancer are the most important tumor antigens recognized by T cells. Among immunologically "cold" tumors, limited recognition of tumor neoantigens results in the absence of a de novo antitumor immune response. These "cold" tumors present a clinical challenge as they are poorly responsive to most immunotherapies, including immune checkpoint inhibitors (ICIs). Radiation therapy (RT) can enhance immune recognition of "cold" tumors, resulting in a more diversified antitumor T-cell response, yet RT alone rarely results in a systemic antitumor immune response. Therefore, a multifunctional bacterial membrane-coated nanoparticle (BNP) composed of an immune activating PC7A/CpG polyplex core coated with bacterial membrane and imide groups to enhance antigen retrieval is developed. This BNP can capture cancer neoantigens following RT, enhance their uptake in dendritic cells (DCs), and facilitate their cross presentation to stimulate an antitumor T-cell response. In mice bearing syngeneic melanoma or neuroblastoma, treatment with BNP+RT results in activation of DCs and effector T cells, marked tumor regression, and tumor-specific antitumor immune memory. This BNP facilitates in situ immune recognition of a radiated tumor, enabling a novel personalized approach to cancer immunotherapy using off-the-shelf therapeutics.
PMID:31523868 | PMC:PMC6810793 | DOI:10.1002/adma.201902626
View details for PubMedID 31523868
-
More
-
Preclinical Characterization of <sup>86/90</sup>Y-NM600 in a Variety of Murine and Human Cancer Tumor Models Journal of nuclear medicine : official publication, Society of Nuclear Medicine
Grudzinski JJ, Hernandez R, Marsh I, Patel RB, Aluicio-Sarduy E, Engle J, Morris Z, Bednarz B, Weichert J
2019 Nov;60(11):1622-1628. doi: 10.2967/jnumed.118.224808. Epub 2019 Apr 6.
-
More
We characterize the in vivo biodistribution and tumor selectivity of 86Y-NM600, a theranostic alkylphosphocholine radiometal chelate with broad tumor selectivity, in a variety of preclinical cancer models. Methods: Mice bearing flank tumors (representative of lung, pancreatic, prostate, liver, skin, and lymphoid cancers) were injected intravenously with 9.25 MBq of 86Y-NM600 and imaged longitudinally over 4-5 d using small-animal PET/CT. Percentage injected activity per gram (%IA/g) for each volume of interest was measured at each time point for the organs of interest. Mice were euthanized after the final time point, and the tumor and organs of interest were counted with an automatic γ-counter. Absorbed doses delivered by 90Y-NM600 per injected activity (Gy/MBq) were estimated. Mice bearing B78 flank tumors were injected with a prescription of 90Y-NM600 that delivered 2.5 Gy of absorbed tumor dose and was compared with an equivalent absorbed dose delivered via external-beam radiotherapy using tumor volume as a measure of response. Histology and complete blood counts were analyzed in naïve C57BL/6 mice that were injected with 9.25 MBq of 90Y-NM600 at 5, 10, and 28 d after injection. Results: PET imaging showed consistent tumor accumulation and retention across all tumor models investigated, with little off-target retention of NM600 except in the liver, as is characteristic of hepatobiliary metabolism. The tumor uptake was highest in the pancreatic and lymphoid cancer models, reaching peak concentrations of 9.34 ± 2.66 %IA/g (n = 3) and 9.10 ± 0.13 %IA/g (n = 3), respectively, at approximately 40-48 h after injection. These corresponded to tumor dose estimates of 2.72 ± 0.33 Gy/MBq and 2.67 ± 0.32 Gy/MBq, respectively. In the toxicity study, there were no visible signs of acute toxicity by histology, and perturbation of hematologic parameters was transient when observed, returning to pretherapy levels after 28 d. Conclusion: NM600 is a theranostic agent with a unique ability to selectively target a variety of cancer types, presenting a unique opportunity for PET image-guided targeted radionuclide therapy and combination with immunotherapies.
PMID:30954941 | DOI:10.2967/jnumed.118.224808
View details for PubMedID 30954941
-
More
-
Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities Brachytherapy
Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS
2019 Mar-Apr;18(2):240. doi: 10.1016/j.brachy.2019.01.004.
-
<sup>90</sup>Y-NM600 targeted radionuclide therapy induces immunologic memory in syngeneic models of T-cell Non-Hodgkin's Lymphoma Communications biology
Hernandez R, Walker KL, Grudzinski JJ, Aluicio-Sarduy E, Patel R, Zahm CD, Pinchuk AN, Massey CF, Bitton AN, Brown RJ, Sondel PM, Morris ZS, Engle JW, Capitini CM, Weichert JP
2019 Feb 26;2:79. doi: 10.1038/s42003-019-0327-4. eCollection 2019.
-
More
Finding improved therapeutic strategies against T-cell Non-Hodgkin's Lymphoma (NHL) remains an unmet clinical need. We implemented a theranostic approach employing a tumor-targeting alkylphosphocholine (NM600) radiolabeled with 86Y for positron emission tomography (PET) imaging and 90Y for targeted radionuclide therapy (TRT) of T-cell NHL. PET imaging and biodistribution performed in mouse models of T-cell NHL showed in vivo selective tumor uptake and retention of 86Y-NM600. An initial toxicity assessment examining complete blood counts, blood chemistry, and histopathology of major organs established 90Y-NM600 safety. Mice bearing T-cell NHL tumors treated with 90Y-NM600 experienced tumor growth inhibition, extended survival, and a high degree of cure with immune memory toward tumor reestablishment. 90Y-NM600 treatment was also effective against disseminated tumors, improving survival and cure rates. Finally, we observed a key role for the adaptive immune system in potentiating a durable anti-tumor response to TRT, especially in the presence of microscopic disease.
PMID:30820474 | PMC:PMC6391402 | DOI:10.1038/s42003-019-0327-4
View details for PubMedID 30820474
-
More
-
International Survey on the Use of Complementary and Alternative Medicines for Common Toxicities of Radiation Therapy Advances in radiation oncology
Lee A, Kuczmarska-Haas A, Macomber MW, Woo K, Freese C, Morris ZS
2018 Oct 5;4(1):134-141. doi: 10.1016/j.adro.2018.09.012. eCollection 2019 Jan-Mar.
-
More
PURPOSE: Complementary and alternative medicines (CAMs) are widely used by patients with cancer. However, little is known about the extent to which these potential remedies are used internationally to treat the most common toxicities of radiation therapy. We report on the results of an international survey that assessed the use of CAMs.
METHODS AND MATERIALS: Surveys were distributed to 1174 practicing radiation oncologists. Questions evaluated the perceptions of CAMs and specific practice patterns for the use of CAM remedies in the treatment of common radiation-induced toxicities (eg, skin, fatigue, nausea, diarrhea, and mucositis/xerostomia). The responses were compared between the groups using the χ2 test and stratified on the basis of provider location, number of years in practice, and perception of CAMs.
RESULTS: A total of 114 radiation oncologists from 29 different countries completed the survey, with a balanced distribution between North American (n = 56) and non-North American (n = 58) providers. Among the responding clinicians, 63% recommended CAMs in their practice. The proportion of clinicians who recommend CAMs for radiation toxicities did not significantly vary when stratified by provider's number of years in practice (P = .23) or location (United States/Canada vs other; P = .74). Overall, providers reported that 29.4% of their patients use CAMs, and 87.7% reported that their practice encouraged or was neutral on CAM use, whereas 12.3% recommended stopping CAMs. The most common sources of patient information on CAMs were the Internet (75.4%), friends (60.5%), and family (58.8%). Clinicians reported the highest use of CAMs for radiation skin toxicity at 66.7%, followed by 48.2% for fatigue, 40.4% for nausea, and 36.8% for mucositis/xerostomia.
CONCLUSIONS: Nearly two-thirds of the surveyed radiation oncologists recommend CAMs for radiation-related toxicities; however, they estimated that less than one third of patients use CAMs for this purpose. This suggests a need for further investigation and perhaps greater patient education on the roles of CAMs in treating radiation toxicities.
PMID:30706021 | PMC:PMC6349625 | DOI:10.1016/j.adro.2018.09.012
View details for PubMedID 30706021
-
More
-
Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities Brachytherapy
Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS
2018 Nov-Dec;17(6):995-1003. doi: 10.1016/j.brachy.2018.07.004. Epub 2018 Aug 2.
-
More
As immunotherapies continue to emerge as a standard component of treatment for a variety of cancers, the imperative for testing these in combination with other standard cancer therapies grows. Radiation therapy may be a particularly well-suited partner for many immunotherapies. By modulating immune tolerance and functional immunogenicity at a targeted tumor site, radiation therapy may serve as a method of in situ tumor vaccination. In situ tumor vaccination is a therapeutic strategy that seeks to convert a patient's own tumor into a nidus for enhanced presentation of tumor-specific antigens in a way that will stimulate and diversify an antitumor T cell response. The mechanisms whereby radiation may impact immunotherapy are diverse and include its capacity to simultaneously elicit local inflammation, temporary local depletion of suppressive lymphocyte lineages, enhanced tumor cell susceptibility to immune response, and immunogenic tumor cell death. Emerging data suggest that each of these mechanisms may display a distinct dose-response profile, making it challenging to maximize each of these effects using external beam radiation. Conversely, the highly heterogenous and conformal dose distribution achieved with brachytherapy may be optimal for enhancing the immunogenic capacity of radiation at a tumor site while minimizing off-target antagonistic effects on peripheral immune cells. Here, we review the immunogenic effects of radiation, summarize the clinical rationale and data supporting the use of radiation together with immunotherapies, and discuss the rationale and urgent need for further preclinical and clinical investigation specifically of brachytherapy in combination with immunotherapies. Harnessing these immunomodulatory effects of brachytherapy may offer solutions to overcome obstacles to the efficacy of immunotherapies in immunologically "cold" tumors while potentiating greater response in the context of immunologically "hot" tumors.
PMID:30078541 | PMC:PMC8292980 | DOI:10.1016/j.brachy.2018.07.004
View details for PubMedID 30078541
-
More
-
Tumor-Specific Inhibition of <em>In Situ</em> Vaccination by Distant Untreated Tumor Sites Cancer immunology research
Morris ZS, Guy EI, Werner LR, Carlson PM, Heinze CM, Kler JS, Busche SM, Jaquish AA, Sriramaneni RN, Carmichael LL, Loibner H, Gillies SD, Korman AJ, Erbe AK, Hank JA, Rakhmilevich AL, Harari PM, Sondel PM
2018 Jul;6(7):825-834. doi: 10.1158/2326-6066.CIR-17-0353. Epub 2018 May 10.
-
More
In situ vaccination is an emerging cancer treatment strategy that uses local therapies to stimulate a systemic antitumor immune response. We previously reported an in situ vaccination effect when combining radiation (RT) with intratumor (IT) injection of tumor-specific immunocytokine (IC), a fusion of tumor-specific antibody and IL2 cytokine. In mice bearing two tumors, we initially hypothesized that delivering RT plus IT-IC to the "primary" tumor would induce a systemic antitumor response causing regression of the "secondary" tumor. To test this, mice bearing one or two syngeneic murine tumors of B78 melanoma and/or Panc02 pancreatic cancer were treated with combined external beam RT and IT-IC to the designated "primary" tumor only. Primary and secondary tumor response as well as animal survival were monitored. Immunohistochemistry and quantitative real-time PCR were used to quantify tumor infiltration with regulatory T cells (Treg). Transgenic "DEREG" mice or IgG2a anti-CTLA-4 were used to transiently deplete tumor Tregs. Contrary to our initial hypothesis, we observed that the presence of an untreated secondary tumor antagonized the therapeutic effect of RT + IT-IC delivered to the primary tumor. We observed reciprocal tumor specificity for this effect, which was circumvented if all tumors received RT or by transient depletion of Tregs. Primary tumor treatment with RT + IT-IC together with systemic administration of Treg-depleting anti-CTLA-4 resulted in a renewed in situ vaccination effect. Our findings show that untreated tumors can exert a tumor-specific, Treg-dependent, suppressive effect on the efficacy of in situ vaccination and demonstrate clinically viable approaches to overcome this effect. Untreated tumor sites antagonize the systemic and local antitumor immune response to an in situ vaccination regimen. This effect is radiation sensitive and may be mediated by tumor-specific regulatory T cells harbored in the untreated tumor sites. Cancer Immunol Res; 6(7); 825-34. ©2018 AACR.
PMID:29748391 | PMC:PMC6030484 | DOI:10.1158/2326-6066.CIR-17-0353
View details for PubMedID 29748391
-
More
-
Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology The Lancet. Oncology
Bristow RG, Alexander B, Baumann M, Bratman SV, Brown JM, Camphausen K, Choyke P, Citrin D, Contessa JN, Dicker A, Kirsch DG, Krause M, Le Q, Milosevic M, Morris ZS, Sarkaria JN, Sondel PM, Tran PT, Wilson GD, Willers H, Wong KS, Harari PM
2018 May;19(5):e240-e251. doi: 10.1016/S1470-2045(18)30096-2.
-
More
The practice of radiation oncology is primarily based on precise technical delivery of highly conformal, image-guided external beam radiotherapy or brachytherapy. However, systematic research efforts are being made to facilitate individualised radiation dose prescriptions on the basis of gene-expressssion profiles that reflect the radiosensitivity of tumour and normal tissue. This advance in precision radiotherapy should complement those benefits made in precision cancer medicine that use molecularly targeted agents and immunotherapies. The personalisation of cancer therapy, predicated largely on genomic interrogation, is facilitating the selection of therapies that are directed against driver mutations, aberrant cell signalling, tumour microenvironments, and genetic susceptibilities. With the increasing technical power of radiotherapy to safely increase local tumour control for many solid tumours, it is an opportune time to rigorously explore the potential benefits of combining radiotherapy with molecular targeted agents and immunotherapies to increase cancer survival outcomes. This theme provides the basis and foundation for this American Society for Radiation Oncology guideline on combining radiotherapy with molecular targeting and immunotherapy agents.
PMID:29726389 | DOI:10.1016/S1470-2045(18)30096-2
View details for PubMedID 29726389
-
More
-
Transcriptional-mediated effects of radiation on the expression of immune susceptibility markers in melanoma Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Werner LR, Kler JS, Gressett MM, Riegert M, Werner LK, Heinze CM, Kern JG, Abbariki M, Erbe AK, Patel RB, Sriramaneni RN, Harari PM, Morris ZS
2017 Sep;124(3):418-426. doi: 10.1016/j.radonc.2017.08.016. Epub 2017 Sep 8.
-
More
BACKGROUND AND PURPOSE: We recently reported a time-sensitive, cooperative, anti-tumor effect elicited by radiation (RT) and intra-tumoral-immunocytokine injection in vivo. We hypothesized that RT triggers transcriptional-mediated changes in tumor expression of immune susceptibility markers at delayed time points, which may explain these previously observed time-dependent effects.
MATERIALS AND METHODS: We examined the time course of changes in expression of immune susceptibility markers following in vitro or in vivo RT in B78 murine melanoma and A375 human melanoma using flow cytometry, immunoblotting, and qPCR.
RESULTS: Flow cytometry and immunoblot revealed time-dependent increases in expression of death receptors and T cell co-stimulatory/co-inhibitory ligands following RT in murine and human melanoma. Using high-throughput qPCR, we observed comparable time courses of RT-induced transcriptional upregulation for multiple immune susceptibility markers. We confirmed analogous changes in B78 tumors irradiated in vivo. We observed upregulated expression of DNA damage response markers days prior to changes in immune markers, whereas phosphorylation of the STAT1 transcription factor occurred concurrently with changes following RT.
CONCLUSION: This study highlights time-dependent, transcription-mediated changes in tumor immune susceptibility marker expression following RT. These findings may help in the design of strategies to optimize sequencing of RT and immunotherapy in translational and clinical studies.
PMID:28893414 | PMC:PMC5626442 | DOI:10.1016/j.radonc.2017.08.016
View details for PubMedID 28893414
-
More
-
Bridging Innovation and Outreach to Overcome Global Gaps in Radiation Oncology Through Information and Communication Tools, Trainee Advancement, Engaging Industry, Attention to Ethical Challenges, and Political Advocacy Seminars in radiation oncology
Dad L, Royce TJ, Morris Z, Moran M, Pawlicki T, Khuntia D, Hardenbergh P, Cummings B, Mayr N, Hu K
2017 Apr;27(2):98-108. doi: 10.1016/j.semradonc.2016.11.002. Epub 2016 Nov 9.
-
More
An evolving paradigm in global outreach in radiation oncology has been the implementation of a more region-specific, needs-based approach to help close the gap in radiation services to low- and middle-income countries through the use of innovative tools in information and communication technology. This report highlights 4 information and communication technology tools in action today: (1) the NCCN Framework for Resource Stratification of NCCN guidelines, (2) ASTRO e-Contouring, (3) i.treatsafely.org, and (4) ChartRounds.com. We also render special consideration to matters related to global outreach that we believe require distinct attention to help us meet the goals established by the 2011 United Nations׳ Declaration on noncommunicable diseases: (1) trainee advancement toward careers in global health, (2) ethical challenges of international outreach, (3) critical importance of political advocacy, and (4) collaboration with Industry.
PMID:28325248 | DOI:10.1016/j.semradonc.2016.11.002
View details for PubMedID 28325248
-
More
-
Supply and Demand for Radiation Oncology in the United States: A Resident Perspective International journal of radiation oncology, biology, physics
Burt LM, Trifiletti DM, Nabavizadeh N, Katz LM, Morris ZS, Royce TJ
2017 Feb 1;97(2):225-227. doi: 10.1016/j.ijrobp.2016.10.038.
-
Online patient information from radiation oncology departments is too complex for the general population Practical radiation oncology
Rosenberg SA, Francis DM, Hullet CR, Morris ZS, Brower JV, Anderson BM, Bradley KA, Bassetti MF, Kimple RJ
2017 Jan-Feb;7(1):57-62. doi: 10.1016/j.prro.2016.07.008. Epub 2016 Aug 1.
-
More
PURPOSE: Nearly two-thirds of cancer patients seek information about their diagnosis online. We assessed the readability of online patient education materials found on academic radiation oncology department Web sites to determine whether they adhered to guidelines suggesting that information be presented at a sixth-grade reading level.
METHODS AND MATERIALS: The Association of American Medical Colleges Web site was used to identify all academic radiation oncology departments in the United States. One-third of these department Web sites were selected for analysis using a random number generator. Both general information on radiation therapy and specific information regarding various radiation modalities were collected. To test the hypothesis that the readability of these online educational materials was written at the recommended grade level, a panel of 10 common readability tests was used. A composite grade level of readability was constructed using the 8 readability measures that provide a single grade-level output.
RESULTS: A mean of 5605 words (range, 2058-12,837) from 30 department Web sites was collected. Using the composite grade level score, the overall mean readability level was determined to be 13.36 (12.83-13.89), corresponding to a collegiate reading level. This was significantly higher than the target sixth-grade reading level (middle school, t (29) = 27.41, P < .001).
CONCLUSIONS: Online patient educational materials from academic radiation oncology Web sites are significantly more complex than recommended by the National Institutes of Health and the Department of Health and Human Services. To improve patients' comprehension of radiation therapy and its role in their treatment, our analysis suggests that the language used in online patient information should be simplified to communicate the information at a more appropriate level.
PMID:27663932 | PMC:PMC5219938 | DOI:10.1016/j.prro.2016.07.008
View details for PubMedID 27663932
-
More
-
Readability of Online Patient Educational Resources Found on NCI-Designated Cancer Center Web Sites Journal of the National Comprehensive Cancer Network : JNCCN
Rosenberg SA, Francis D, Hullett CR, Morris ZS, Fisher MM, Brower JV, Bradley KA, Anderson BM, Bassetti MF, Kimple RJ
2016 Jun;14(6):735-40. doi: 10.6004/jnccn.2016.0075.
-
More
BACKGROUND: The NIH and Department of Health & Human Services recommend online patient information (OPI) be written at a sixth grade level. We used a panel of readability analyses to assess OPI from NCI-Designated Cancer Center (NCIDCC) Web sites.
METHODS: Cancer.gov was used to identify 68 NCIDCC Web sites from which we collected both general OPI and OPI specific to breast, prostate, lung, and colon cancers. This text was analyzed by 10 commonly used readability tests: the New Dale-Chall Readability Formula, Flesch Reading Ease scale, Flesch-Kinaid Grade Level, FORCAST scale, Fry Readability Graph, Simple Measure of Gobbledygook test, Gunning Frequency of Gobbledygook index, New Fog Count, Raygor Readability Estimate Graph, and Coleman-Liau Index. We tested the hypothesis that the readability of NCIDCC OPI was written at the sixth grade level. Secondary analyses were performed to compare readability of OPI between comprehensive and noncomprehensive centers, by region, and to OPI produced by the American Cancer Society (ACS).
RESULTS: A mean of 30,507 words from 40 comprehensive and 18 noncomprehensive NCIDCCs was analyzed (7 nonclinical and 3 without appropriate OPI were excluded). Using a composite grade level score, the mean readability score of 12.46 (ie, college level: 95% CI, 12.13-12.79) was significantly greater than the target grade level of 6 (middle-school: P<.001). No difference between comprehensive and noncomprehensive centers was identified. Regional differences were identified in 4 of the 10 readability metrics (P<.05). ACS OPI provides easier language, at the seventh to ninth grade level, across all tests (P<.01).
CONCLUSIONS: OPI from NCIDCC Web sites is more complex than recommended for the average patient.
PMID:27283166 | PMC:PMC7236813 | DOI:10.6004/jnccn.2016.0075
View details for PubMedID 27283166
-
More
-
Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers Cancer
Morris ZS, Saha S, Magnuson WJ, Morris BA, Borkenhagen JF, Ching A, Hirose G, McMurry V, Francis DM, Harari PM, Chappell R, Tsuji S, Ritter MA
2016 Aug 15;122(16):2487-95. doi: 10.1002/cncr.30079. Epub 2016 May 20.
-
More
BACKGROUND: Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are commonly used antihypertensive medications that have been reported to affect aberrant angiogenesis and the dysregulated inflammatory response. Because of such mechanisms, it was hypothesized that these medications might affect the tumor response to neoadjuvant radiation in patients with rectal cancer.
METHODS: One hundred fifteen patients who were treated with neoadjuvant radiation at the University of Wisconsin (UW) between 1999 and 2012 were identified. Univariate analyses were performed with anonymized patient data. In a second independent data set, 186 patients with rectal cancer who were treated with neoadjuvant radiation at the Queen's Medical Center of the University of Hawaii (UH) between 1995 and 2010 were identified. These data were independently analyzed as before. Multivariate analyses were performed with aggregate data.
RESULTS: Among patients taking ACEIs/ARBs in the UW data set, a significant 3-fold increase in the rate of pathologic complete response (pCR) to neoadjuvant therapy (52% vs 17%, P = .001) was observed. This finding was confirmed in the UH data set, in which a significant 2-fold-increased pCR rate (24% vs 12%, P = .03) was observed. Identified patient and treatment characteristics were otherwise balanced between patients taking and not taking ACEIs/ARBs. No significant effect was observed on pCR rates with other medications, including statins, metformin, and aspirin. Multivariate analyses of aggregate data identified ACEI/ARB use as a strong predictor of pCR (odds ratio, 4.02; 95% confidence interval, 2.06-7.82; P < .001).
CONCLUSIONS: The incidental use of ACEIs/ARBs among patients with rectal cancer is associated with a significantly increased rate of pCR after neoadjuvant treatment. Cancer 2016;122:2487-95. © 2016 American Cancer Society.
PMID:27203227 | PMC:PMC4998053 | DOI:10.1002/cncr.30079
View details for PubMedID 27203227
-
More
-
In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments Cancer research
Morris ZS, Guy EI, Francis DM, Gressett MM, Werner LR, Carmichael LL, Yang RK, Armstrong EA, Huang S, Navid F, Gillies SD, Korman A, Hank JA, Rakhmilevich AL, Harari PM, Sondel PM
2016 Jul 1;76(13):3929-41. doi: 10.1158/0008-5472.CAN-15-2644. Epub 2016 May 6.
-
More
Interest in combining radiotherapy and immune checkpoint therapy is growing rapidly. In this study, we explored a novel combination of this type to augment antitumor immune responses in preclinical murine models of melanoma, neuroblastoma, and head and neck squamous cell carcinoma. Cooperative effects were observed with local radiotherapy and intratumoral injection of tumor-specific antibodies, arising in part from enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). We could improve this response by combining radiation with intratumoral injection of an IL2-linked tumor-specific antibody (termed here an immunocytokine), resulting in complete regression of established tumors in most animals associated with a tumor-specific memory T-cell response. Given the T-cell response elicited by combined local radiation and intratumoral immunocytokine, we tested the potential benefit of adding this treatment to immune checkpoint blockade. In mice bearing large primary tumors or disseminated metastases, the triple-combination of intratumoral immunocytokine, radiation, and systemic anti-CTLA-4 improved primary tumor response and animal survival compared with combinations of any two of these three interventions. Taken together, our results show how combining radiation and intratumoral immunocytokine in murine tumor models can eradicate large tumors and metastases, eliciting an in situ vaccination effect that can be leveraged further by T-cell checkpoint blockade, with immediate implications for clinical evaluation. Cancer Res; 76(13); 3929-41. ©2016 AACR.
PMID:27197149 | PMC:PMC4930687 | DOI:10.1158/0008-5472.CAN-15-2644
View details for PubMedID 27197149
-
More
-
Results of the 2013-2015 Association of Residents in Radiation Oncology Survey of Chief Residents in the United States International journal of radiation oncology, biology, physics
Nabavizadeh N, Burt LM, Mancini BR, Morris ZS, Walker AJ, Miller SM, Bhavsar S, Mohindra P, Kim MB, Kharofa J, Committee AE
2016 Feb 1;94(2):228-34. doi: 10.1016/j.ijrobp.2015.10.014. Epub 2015 Oct 22.
-
More
PURPOSE: The purpose of this project was to survey radiation oncology chief residents to define their residency experience and readiness for independent practice.
METHODS AND MATERIALS: During the academic years 2013 to 2014 and 2014 to 2015, the Association of Residents in Radiation Oncology (ARRO) conducted an electronic survey of post-graduate year-5 radiation oncology residents in the United States during the final 3 months of training. Descriptive statistics are reported.
RESULTS: Sixty-six chief residents completed the survey in 2013 to 2014 (53% response rate), and 69 completed the survey in 2014 to 2015 (64% response rate). Forty to 85% percent of residents reported inadequate exposure to high-dose rate and low-dose rate brachytherapy. Nearly all residents in both years (>90%) reported adequate clinical experience for the following disease sites: breast, central nervous system, gastrointestinal, genitourinary, head and neck, and lung. However, as few as 56% reported adequate experience in lymphoma or pediatric malignancies. More than 90% of residents had participated in retrospective research projects, with 20% conducting resident-led prospective clinical trials and 50% conducting basic science or translational projects. Most chief residents reported working 60 or fewer hours per week in the clinical/hospital setting and performing fewer than 15 hours per week tasks that were considered to have little or no educational value. There was more than 80% compliance with Accreditation Council for Graduate Medical Education (ACGME) work hour limits. Fifty-five percent of graduating residents intended to join an established private practice group, compared to 25% who headed for academia. Residents perceive the job market to be more competitive than previous years.
CONCLUSIONS: This first update of the ARRO chief resident survey since the 2007 to 2008 academic year documents US radiation oncology residents' experiences and conditions over a 2-year period. This analysis may serve as a valuable tool for those seeking to improve training of the next generation of oncology leaders.
PMID:26853332 | DOI:10.1016/j.ijrobp.2015.10.014
View details for PubMedID 26853332
-
More
-
Education and Training Needs in Radiation Oncology in India: Opportunities for Indo-US Collaborations International journal of radiation oncology, biology, physics
Grover S, Chadha M, Rengan R, Williams TR, Morris ZS, Morgan AL, Tripuraneni P, Hu K, Viswanathan AN
2015 Dec 1;93(5):957-60. doi: 10.1016/j.ijrobp.2015.08.009. Epub 2015 Aug 7.
-
More
PURPOSE: To conduct a survey of radiation oncologists in India, to better understand specific educational needs of radiation oncology in India and define areas of collaboration with US institutions.
METHODS AND MATERIALS: A 20-question survey was distributed to members of the Association of Indian Radiation Oncologists and the Indian Brachytherapy Society between November 2013 and May 2014.
RESULTS: We received a total of 132 responses. Over 50% of the physicians treat more than 200 patients per day, use 2-dimensional or 3-dimensional treatment planning techniques, and approximately 50% use image guided techniques. For education needs, most respondents agreed that further education in intensity modulated radiation therapy, image guided radiation therapy, stereotactic radiation therapy, biostatistics, and research methods for medical residents would be useful areas of collaboration with institutions in the United States. Other areas of collaboration include developing a structured training module for nursing, physics training, and developing a second-opinion clinic for difficult cases with faculty in the United States.
CONCLUSION: Various areas of potential collaboration in radiation oncology education were identified through this survey. These include the following: establishing education programs focused on current technology, facilitating exchange programs for trainees in India to the United States, promoting training in research methods, establishing training modules for physicists and oncology nurses, and creating an Indo-US. Tumor Board. It would require collaboration between the Association of Indian Radiation Oncologists and the American Society for Radiation Oncology to develop these educational initiatives.
PMID:26581132 | DOI:10.1016/j.ijrobp.2015.08.009
View details for PubMedID 26581132
-
More
-
Pan-HER Inhibitor Augments Radiation Response in Human Lung and Head and Neck Cancer Models Clinical cancer research : an official journal of the American Association for Cancer Research
Francis DM, Huang S, Armstrong EA, Werner LR, Hullett C, Li C, Morris ZS, Swick AD, Kragh M, Lantto J, Kimple RJ, Harari PM
2016 Feb 1;22(3):633-43. doi: 10.1158/1078-0432.CCR-15-1664. Epub 2015 Sep 29.
-
More
PURPOSE: Aberrant regulation of the EGF receptor family (EGFR, HER2, HER3, HER4) contributes to tumorigenesis and metastasis in epithelial cancers. Pan-HER represents a novel molecular targeted therapeutic composed of a mixture of six monoclonal antibodies against EGFR, HER2, and HER3.
EXPERIMENTAL DESIGN: In the current study, we examine the capacity of Pan-HER to augment radiation response across a series of human lung and head and neck cancers, including EGFR inhibitor-resistant cell lines and xenografts.
RESULTS: Pan-HER demonstrates superior antiproliferative and radiosensitizing impact when compared with cetuximab. The mechanisms underlying these effects appear to involve attenuation of DNA damage repair, enhancement of programmed cell death, cell-cycle redistribution, and induction of cellular senescence. Combined treatment of Pan-HER with single or fractionated radiation in human tumor xenografts reveals a potent antitumor and regrowth delay impact compared with Pan-HER or radiation treatment alone.
CONCLUSIONS: These data highlight the capacity of Pan-HER to augment radiation response in lung and head and neck cancer models and support investigation of Pan-HER combined with radiation as a promising clinical therapeutic strategy.
PMID:26420857 | DOI:10.1158/1078-0432.CCR-15-1664
View details for PubMedID 26420857
-
More
-
Impact of a Contralateral Tumor Nodule on Survival in Non-Small-Cell Lung Cancer Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer
Morris ZS, Cannon DM, Morris BA, Bentzen SM, Kozak KR
2015 Nov;10(11):1608-15. doi: 10.1097/JTO.0000000000000655.
-
More
INTRODUCTION: Contralateral lung tumors in non-small-cell lung cancer (NSCLC) are classified as stage M1a yet may represent hematogenous metastases or synchronous primary tumors. The impact of these tumors on overall survival (OS) is poorly understood. Here, we aim to determine whether NSCLC patients with M1a disease due only to a contralateral tumor nodule exhibit a favorable prognosis relative to other M1a or M1b patients.
METHODS: Retrospective evaluation of the impact of contralateral tumor nodules on OS in NSCLC stratified by primary tumor size and N stage attained from Surveillance, Epidemiology, and End Results database.
RESULTS: Of 173,640 patients, 5161 M1a-contra patients were identified. Median and 3-year OS for these patients exceeded that of patients with M1b (p < 0.0001) or other M1a disease (p < 0.0001). Primary tumor size and N stage were strongly associated with OS in M1a-contra patients. Three-year OS demonstrated a delayed convergence between M1a-contra and other M1a patients with primary tumors greater than or equal to 3 cm or mediastinal lymph node involvement. Proportional hazard modeling indicated that T1-2N0-1M1a-contra patients exhibit OS not significantly different (p = 0.258) from that predicted with comparable T and N stage disease plus a second early-stage primary.
CONCLUSIONS: Contralateral tumors in NSCLC carry a more favorable prognosis than other M1a or M1b disease. Primary tumor size and N stage may help distinguish M1a-contra patients with hematogenous metastasis from those with a synchronous, second primary.
PMID:26317917 | PMC:PMC4636460 | DOI:10.1097/JTO.0000000000000655
View details for PubMedID 26317917
-
More
-
NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy Frontiers in immunology
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM
2015 Jul 27;6:368. doi: 10.3389/fimmu.2015.00368. eCollection 2015.
-
More
Natural killer (NK) cells play a major role in cancer immunotherapies that involve tumor-antigen targeting by monoclonal antibodies (mAbs). NK cells express a variety of activating and inhibitory receptors that serve to regulate the function and activity of the cells. In the context of targeting cells, NK cells can be "specifically activated" through certain Fc receptors that are expressed on their cell surface. NK cells can express FcγRIIIA and/or FcγRIIC, which can bind to the Fc portion of immunoglobulins, transmitting activating signals within NK cells. Once activated through Fc receptors by antibodies bound to target cells, NK cells are able to lyse target cells without priming, and secrete cytokines like interferon gamma to recruit adaptive immune cells. This antibody-dependent cell-mediated cytotoxicity (ADCC) of tumor cells is utilized in the treatment of various cancers overexpressing unique antigens, such as neuroblastoma, breast cancer, B cell lymphoma, and others. NK cells also express a family of receptors called killer immunoglobulin-like receptors (KIRs), which regulate the function and response of NK cells toward target cells through their interaction with their cognate ligands that are expressed on tumor cells. Genetic polymorphisms in KIR and KIR-ligands, as well as FcγRs may influence NK cell responsiveness in conjunction with mAb immunotherapies. This review focuses on current therapeutic mAbs, different strategies to augment the anti-tumor efficacy of ADCC, and genotypic factors that may influence patient responses to antibody-dependent immunotherapies.
PMID:26284063 | PMC:PMC4515552 | DOI:10.3389/fimmu.2015.00368
View details for PubMedID 26284063
-
More
-
Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Morris ZS, Weichert JP, Saker J, Armstrong EA, Besemer A, Bednarz B, Kimple RJ, Harari PM
2015 Sep;116(3):504-9. doi: 10.1016/j.radonc.2015.06.015. Epub 2015 Jun 26.
-
More
BACKGROUND AND PURPOSE: CLR1404 is a phospholipid ether that exhibits selective uptake and retention in malignant tissues. Radiolabeled CLR1404 enables tumor-specific positron-emission tomography (PET) imaging ((124)I) and targeted delivery of ionizing radiation ((131)I). Here we describe the first preclinical studies of this diapeutic molecule in head and neck cancer (HNC) models.
MATERIAL AND METHODS: Tumor-selective distribution of (124)I-CLR1404 and therapeutic efficacy of (131)I-CLR1404 were tested in HNC cell lines and patient-derived xenograft tumor models. Monte Carlo dose calculations and (124)I-CLR1404 PET/CT imaging were used to examine (131)I-CLR1404 dosimetry in preclinical HNC tumor models.
RESULTS: HNC tumor xenograft studies including patient-derived xenografts demonstrate tumor-selective uptake and retention of (124)I-CLR1404 resulting in a model of highly conformal dose distribution for (131)I-CLR1404. We observe dose-dependent response to (131)I-CLR1404 with respect to HNC tumor xenograft growth inhibition and this effect is maintained together with external beam radiation.
CONCLUSIONS: We confirm the utility of CLR1404 for tumor imaging and treatment of HNC. This promising agent warrants further investigation in a developing phase I trial combining (131)I-CLR1404 with reduced-dose external beam radiation in patients with loco-regionally recurrent HNC.
PMID:26123834 | PMC:PMC4609259 | DOI:10.1016/j.radonc.2015.06.015
View details for PubMedID 26123834
-
More
-
Delivery of definitive dose external beam radiation in close proximity to an implanted deep brain stimulator Practical radiation oncology
Borkenhagen JF, Morris ZS, Hoberg JR, Kozak KR, Shapiro LQ
2014 Sep-Oct;4(5):294-297. doi: 10.1016/j.prro.2013.10.003. Epub 2013 Nov 21.
-
Interaction of radiation therapy with molecular targeted agents Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Morris ZS, Harari PM
2014 Sep 10;32(26):2886-93. doi: 10.1200/JCO.2014.55.1366. Epub 2014 Aug 11.
-
More
The development of molecular targeted therapeutics in oncology builds on many years of scientific investigation into the cellular mechanics of malignant transformation and progression. The past two decades have brought an accelerating pace to the clinical investigation of new molecular targeted agents, particularly in the setting of metastatic disease. The integration of molecular targeted agents into phase III clinical trial design has lagged in the curative treatment setting, particularly in combination with established therapeutic modalities such as radiation. In this review, we discuss the interaction of radiation and molecular targeted therapeutics. The dynamics of cellular and tumor response to radiation offer unique opportunities for beneficial interplay with molecular targeted agents that may go unrecognized with conventional screening and monotherapy clinical testing of novel agents. By using epidermal growth factor receptor (EGFR) as a primary example, we discuss recent clinical studies that illustrate the potential synergy of molecular targeted agents with radiation and highlight the clinical value of such interactions. For various molecular targeted agents, their greatest clinical impact may rest in combination with radiation, and efforts to facilitate systematic investigation of this approach appear highly warranted.
PMID:25113770 | PMC:PMC4152717 | DOI:10.1200/JCO.2014.55.1366
View details for PubMedID 25113770
-
More
Contact Information
Zachary Morris, MD, PhD
1111 Highland Avenue, 3131 WIMR,Madison, WI 53705
